24+ SAMPLE Clinical Trial Agreement
-

Accelerated Clinical Trial Agreement
download now -

Commercial Clinical Trial Agreement
download now -
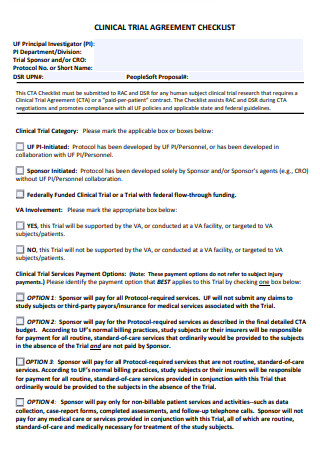
Clinical Trial Agreement Checklist
download now -

Clinical Trial Agreement
download now -

Standard Clinical Trial Agreement
download now -

Clinical Trial Agreement Consideration for Investigaton
download now -

Sample Clinical Trial Agreement
download now -

Clinical Trial Agreement Template
download now -

Final Clinical Trial Agreement
download now -

Initiated Clinical Trial Agreement
download now -

Master Clinical Trial Agreement
download now -

Model Clinical Trial Agreement
download now -

Simple Clinical Trial Agreement
download now -

Clinical Trial Agreement Example
download now -

Health Clinical Trial Agreement Requirements
download now -

Standard Sub-Site Clinical Trial Agreement
download now -
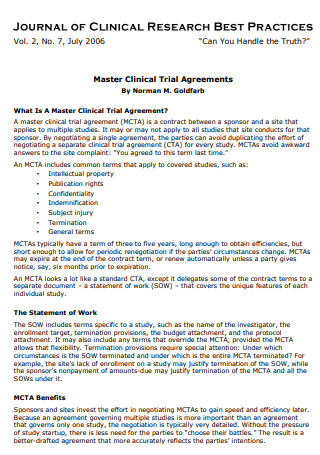
Formal Clinical Trial Agreement
download now -

Clinical Trial Research Agreement
download now -

Clinical Trial Confidentiality Agreement
download now -

Industry Sponsored Clinical Trial Agreement
download now -
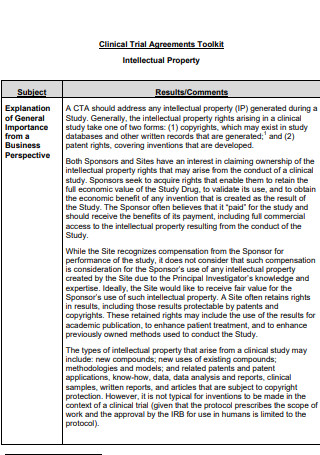
Clinical Trial Agreements Toolkit
download now -

Clinical Trial Agreement for Pharmaceutical
download now -

Clinical Research Organisation Model Clinical Trial Agreement
download now -

Health Center Clinical Trial Agreement
download now -

Guideline of Clinical Trial Agreements in Research
download now
FREE Clinical Trial Agreement s to Download
24+ SAMPLE Clinical Trial Agreement
What is a Clinical Trial?
What is a Clinical Trial Agreement?
What Should be Included in a Clinical Trial Agreement?
What are the Phases of a Clinical Trial?
How to Write an Effective Clinical Trial Agreement
FAQs
Why do people participate in a clinical trial?
What are the different types of clinical trial agreements?
How are participants of a clinical trial chosen?
What is a Clinical Trial?
By definition, a clinical trial refers to the experiments or observations done in clinical research. The purpose of a clinical trial is to let the researchers know if a new medical breakthrough (such as a drug, treatment method, or vaccine) is safe and effective for use on people. People who are willing to participate will volunteer to take part in a clinical trial. But before they can do so, a clinical trial must be approved by the appropriate health and ethics committee in the region where the approval of a medical breakthrough is sought after.
Some clinical trials can also be used to test new ways to detect disease much earlier, sometimes even before symptoms can manifest. It may also look at how to give people who are living with a chronic health problem or a life-threatening disease a better quality of life.
What is a Clinical Trial Agreement?
A clinical trial agreement is a legal document that serves the purpose of managing the relationship between the institution that is conducting the clinical study in a region in order for the results to be provided and published, and the sponsor of the trial that usually provides the treatment drug or vaccine to be tested, or the new treatment methods and clinical interventions to be studied. A clinical trial agreement also outlines the essential details of what the clinical trial will cover and will also clearly spell out the formal understanding that is reached between the parties involved in the study. The agreement document should also contain the financial and legal terms of the study.
What Should be Included in a Clinical Trial Agreement?
Here are the essential elements that should be included in a clinical trial agreement:
What are the Phases of a Clinical Trial?
Clinical trials have stages or phases. The early phase usually looks to test whether the drug, vaccine, or treatment method is safe for human use, and the side effects seen are minimal or none. The later phase of the clinical trials usually looks if there are many ways of having better treatments than the one that is currently available.
Some clinical trials have 4 phases while some have three. Here are the stages of a clinical trial:
How to Write an Effective Clinical Trial Agreement
Here are the necessary steps that should be taken when you begin writing a clinical trial agreement document:
1. Identify the parties involved in the agreement.
In a clinical trial agreement, the parties involved are the sponsors of the study, the institute that will be conducting the clinical trial, and the participants of the study that are located in a specific region. It is also important to identify each of their respective roles in the trial. The role of the sponsors of the study is to provide the drug or the device that is to be tested throughout the duration of the clinical trial. The role of the institute is to perform the studies on the participants, observing them carefully and continuously and noting any significant events that occur during each of the different phases of the trial.
2. Write down the essential elements of the agreement.
In this step, begin writing down the main body of the agreement, along with its essential elements. Keep in mind that a clinical trial agreement should be describing and acknowledging the responsibilities of the parties involved, terms of collaboration, the description of the project/study that is to be conducted, the terms concerning the study’s publication and intellectual property, and the terms regarding the payment and reimbursement of the institution that conducts the clinical study.
3. Write down the elements/clauses that can be used in case something goes wrong.
An essential part of the clinical trial agreement (or any agreement) is also writing down the elements or clauses that can be referenced by all parties involved in case something goes awfully wrong throughout the project. The elements refer to the following: terms regarding insurance or indemnification, guidelines to be used for resolving disputes, coverages in case the subject gets injured during the trials, grounds for terminating the ongoing agreement or contract, and the terms to be referenced for possible amendment of the terms listed in the agreement or contract.
4. Verify the document.
In this step, it is important to go through the agreement once or multiple times and give it a proper verification process after producing your initial draft. Make sure that nothing of importance is left out, and nothing unnecessary to the agreement or project is included in the document. The process of editing and proofreading consists of the examination of the entire material, structure, clarity, and style. It is important to review the document for the purpose of monitoring, adverse event reporting, accountability purposes, and checking for negotiable terms or clauses. It is also recommended that you submit the paper over to a legal professional for evaluation after thorough rewriting and editing. It is always advisable to seek a legal professional’s advice to help you find ways to produce effective and legal documents.
FAQs
Why do people participate in a clinical trial?
There are various reasons why some people choose to participate in a clinical trial. Some people choose to join a clinical trial because they have tried many different treatments for their current health problem but have found that none of them work. They could also join a trial because of the zero availability of treatments for their condition. Participating in a clinical trial also serves as a way for them to play a more active role in their health care. Whatever the reason they have, they automatically become a partner of a scientific breakthrough.
What are the different types of clinical trial agreements?
There are 4 different types of clinical trial agreements, namely:
- Agreement with an industry sponsor – this type of clinical trial agreement is required whenever the drug or medical device to be studied already has provided funding beforehand.
- Investigator initiated clinical trial – this type of clinical trial is very similar to the first one, in that it is required whenever an investigator supplies the drug or device to be provided along with the necessary funding.
- Agreement with an academic institution – This type of clinical trial agreement is either a sub-contract or a sub-award that consists of a lead site recruiting more sub-sites that can serve as the location of the clinical trial.
- Sponsored Projects – this type of agreement or project involves negotiating, reviewing, and executing legal agreements from outside funding sources. Coordination between all the parties involved in the agreement is important for the success of the agreement.
How are participants of a clinical trial chosen?
After the participant gives their consent to the researcher, he/she then undergoes a screening to see if the participant meets the criteria to participate or if there is anything that can prevent them from doing so. Examples of criteria for clinical trial participation might include age, stage of disease, gender, genetic profile, family history, and whether or not he/she has a study partner who can accompany them to future visits. Criteria that can exclude the person from participating might include factors such as specific health conditions or medications that could interfere with the treatment being tested. However, one should keep in mind that different clinical trials follow different criteria, so getting excluded from a certain study does not mean that you are automatically not qualified for future different ones.
An effectively written clinical trial agreement legally binds the parties involved (the sponsor of the study, the researchers or scientists who will conduct the trials, and the participants of the study) and protects them from any unforeseen repercussions or incidents that may arise during the duration of the study. It is also important to have an agreement for proper risk allocation, fund allocation, responsibilities, and obligations allocation, and to protect the academic, intellectual property, legal, and integrity rights of the parties involved. In this article, examples are posted above so you can have a reference should you be required to draft one in the future.
