Balancing Chemical Equations Worksheet Samples
-

Balancing Equations Worksheet With Answer PDF
download now -

Balancing Chemical Equations Worksheet With Answers
download now -

Balancing Chemical Equations Worksheet PDF
download now -

Balancing Chemical Equations Worksheet Easy
download now -

Balancing Equations Worksheet and Key Template
download now -
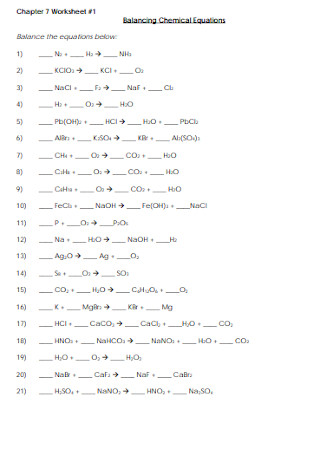
Basic Balancing Chemical Equations Worksheet Template
download now -

Sample Writing and Balancing Equations Worksheet
download now -

Balancing Chemical Equations Worksheet
download now -

Balancing the Given Chemical Equations Worksheet
download now -
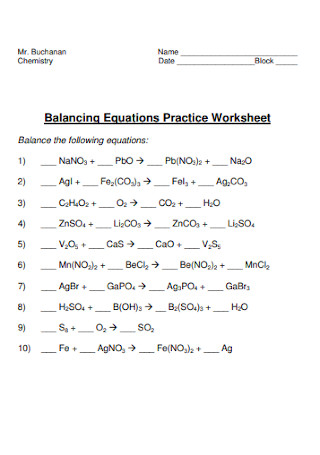
Balancing Equations Practice Worksheet
download now -
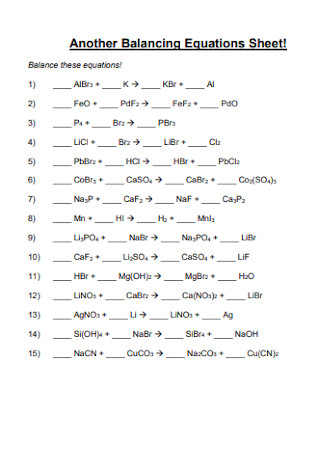
Another Balancing Equations Sheet!
download now -

Simple Balancing Equations Worksheet
download now -

Formal Balancing Chemical Equations Worksheet
download now -

Sample Balancing Chemical Equations Worksheet Example
download now -

Balancing Chemical Equations Reactions Worksheet
download now -

Formal Balancing Equations Worksheet
download now -

Balancing Equations Worksheet Format
download now -
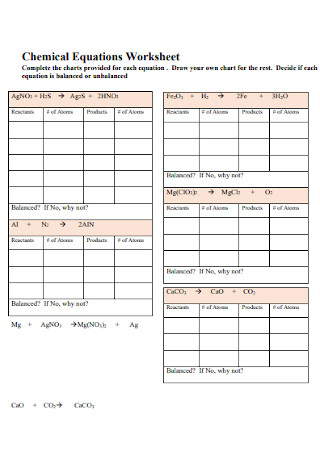
Printable Balancing Chemical Equations Worksheet
download now -
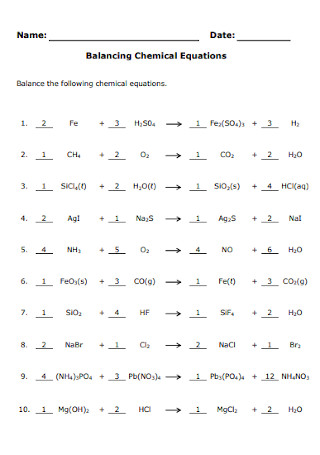
Balancing Chemical Equations Worksheet Template
download now -

Balancing Equations Worksheets in PDF
download now -

Balancing Blackline Master Equations Worksheet
download now -

Balancing Chemistry Chemical Equations Worksheet
download now -

Professional Balancing Chemical Equations Worksheet Example
download now -

Basic Balancing Equations Worksheet Example
download now
FREE Balancing Chemical Equations Worksheet s to Download
Balancing Chemical Equations Worksheet Format
Balancing Chemical Equations Worksheet Samples
What is Balancing Chemical Equations Worksheet?
Uses of the Worksheet
Useful Tips and Tricks
How to Introduce Chemical Reactions
What triggers a chemical reaction?
What are the different kinds of chemical reactions?
Why should both sides of chemical equations be balanced?
What is the function of the parentheses in chemical equations
What does the triangle stand for in chemical equations?
How do you identify reactants and products in the Balancing Chemical Equations Worksheet?
Why is it important to balance equations in the Balancing Chemical Equations Worksheet?
How do coefficients help in the Balancing Chemical Equations Worksheet?
What is the role of the conservation of mass in the Balancing Chemical Equations Worksheet?
What is a systematic approach to solving the Balancing Chemical Equations Worksheet?
What common mistakes should you avoid in the Balancing Chemical Equations Worksheet?
What tips are useful for beginners on the Balancing Chemical Equations Worksheet?
How does stoichiometry help with the Balancing Chemical Equations Worksheet?
What is the first step in the Balancing Chemical Equations Worksheet?
Why is it important to double-check your work on the Balancing Chemical Equations Worksheet?
How do you handle complex equations on the Balancing Chemical Equations Worksheet?
How does the Balancing Chemical Equations Worksheet improve your understanding of reactions?
How can you verify an equation is balanced on the Balancing Chemical Equations Worksheet?
How does practicing with the Balancing Chemical Equations Worksheet enhance problem-solving skills?
Download Balancing Chemical Equations Worksheet Bundle
Balancing Chemical Equations Worksheet Format
Introduction
Provide a brief introduction to the concept of balancing chemical equations. Explain the importance of maintaining the law of conservation of mass and give an overview of the steps involved in balancing equations.
Instructions
- Identify the Reactants and Products: List all reactants on the left side and products on the right side of the equation.
- Write the Unbalanced Equation: Write the chemical formulas of the reactants and products.
- Count Atoms of Each Element: Count the number of atoms of each element on both sides of the equation.
- Balance One Element at a Time: Use coefficients to balance the number of atoms of each element.
- Check Your Work: Ensure that the number of atoms of each element is the same on both sides of the equation.
Practice Problems
- Example 1: H2+O2→H2OH2+O2→H2O
- Unbalanced: H2+O2→H2OH2+O2→H2O
- Balanced: 2H2+O2→2H2O2H2+O2→2H2O
- Example 2: C3H8+O2→CO2+H2OC3H8+O2→CO2+H2O
- Unbalanced: C3H8+O2→CO2+H2OC3H8+O2→CO2+H2O
- Balanced: C3H8+5O2→3CO2+4H2OC3H8+5O2→3CO2+4H2O
(Continue with more examples as needed.)
Tips
- Always start by balancing elements that appear in only one reactant and one product.
- Balance polyatomic ions as a whole if they appear unchanged on both sides of the equation.
Conclusion
Reiterate the importance of practice in mastering balancing equations and encourage students to solve additional problems for better understanding.
What is Balancing Chemical Equations Worksheet?
A Balancing Chemical Equations Worksheet is a learning tool used to practice the process of balancing chemical equations. Balancing equations ensures that the same number of atoms for each element is present on both sides of the equation, reflecting the law of conservation of mass. This worksheet typically includes unbalanced equations, allowing students to apply coefficients and balance them correctly, aiding in the understanding of chemical reactions and stoichiometry.
For instance, photosynthetic plants convert sunlight, carbon dioxide (CO2), and water (H2O) into sugar (C6H12O6) and oxygen (O2). On one side of the equation (reactant), there is 6CO2 + 6H2O. On the other side (product), there is C6H12O6 + O2. What this shows is that the number of elements (like C, H, and O) on one side should be equal to the number of the corresponding elements on the other. No new element is introduced or discarded. Because chemistry is very particular about the details, you have to account for every element in the equation. Carbon dioxide (CO2) is very different from carbon monoxide (CO). The latter can poison and kill a person.
Uses of the Worksheet
As teachers and educators, we can find several uses of balancing chemical equation worksheets in our class. We can use them to test the students’ learning after a discussion. We can use worksheets as props for another event or activity that is relevant to a chemistry lesson.
Useful Tips and Tricks
While there is no shortcut to learning, your students can keep practicing how to balance chemical equations. When they do, they can develop their own styles and approach to solving balancing problems. What if some of your students are having a harder time understanding the lesson? You can help them by giving them advice on how they can solve the equation.
Write Neatly
There would be a lot of letters and numbers involved. If your student seems to write all over the place, he or she might be confused in the middle of solving. If you had just introduced the lesson to the class, show them how to solve one step at a time. For example, every change they make in the numbers can be done per line. They should remember to account for all the numbers that they write. Counting the elements correctly might seem easy, but it will be more challenging when the solution paper is messy.
Take it Slow
Instruct your students to take it slow until they get the hang of balancing chemical equations. They can start by writing the bare equation first. Below the given equation, they can list the elements that are included on both sides. They should also write how many elements there are for each side. Students can begin by balancing all other elements individually while leaving the H and the O at the end. You may also see Home Budget WorkSheet
Trial and Error
Assure your students that mastering how to balancing chemical equations takes time. Make it a teaching strategy to encourage the students to explore solutions. They can begin by trial and error. They can start with the smallest possible number. If that number doesn’t fit, they can go up by single increments. They can do this until they balanced both sides of the equation. Eventually, the students can find their own technique in solving the problem. You may also see Monthly WorkSheet
How to Introduce Chemical Reactions
Understandably, it can be hard to immediately grasp the concept of balancing chemical equations. This, like several lessons in chemistry class, involves unseen forces. The students cannot see atoms, elements, or their interaction. This is usually what makes teaching the subject harder. How do you make your students understand something that they cannot see, smell, hear, or touch? While other teachers would begin with introducing the lesson in a lecture, why not start with its application?
Step 1: Prepare an Experiment
Chemistry experiments involve reactions that aren’t unlike magic. There are color shifts, mini explosions, and other spectacles that will captivate and amaze an audience. You can take advantage of this universal fancy for a good show. Before you begin a lesson about chemical reactions, you can prepare a little show or experiment relating to the lesson. After you showed your students the reaction, you can begin explaining the principle behind that phenomenon. You may also see Budget WorkSheet
Step 2: Show a Short Clip or Video
If an experiment isn’t doable, you can look to the Internet for help. Maybe there is a relevant video or show that shows how your lesson is applied. If you are feeling creative, you can also create your own video project for the students to watch in class. You can schedule the first five minutes or so of the class watching how today’s lesson is used in real-life. One of the challenges of chemistry and other science teachers is to show that what they are teaching is relevant to the students. By showing a video or a clip about the chemical reaction, those chemical equations that you are about to show on the board or screen wouldn’t seem so alien to the students. You may also see Travel Budget WorkSheet
Step 3: Distribute Chemical Equation Worksheets
Before you come to class, print an adequate number of copies of chemical equation worksheets. You can make your own worksheets so that the content of the sheets are relevant to the upcoming class lecture. If your students are just starting to be acquainted with balancing chemical equations, you can choose and download a template from the worksheets in this article. While in class, your students can also follow your discussions through their worksheets. You can use these sheets as test papers that you can collect at the end of the class. By doing so, you can check whether your students have understood what you were discussing. You may also see Household Budget WorkSheet
Step 4: Start Your Lecture
After you have let your students witnessed, through experiments or videos, how several chemical reactions look like, it’s time to talk about why these reactions happen. Your students can follow through with the lecture better if you put this step after your little class show. This is because they have seen for themselves what those elements and compounds do. It is still important to give in-depth treatment to the lecture material. The relationship of different chemicals can’t be concluded from how their interaction looks like. There is a deeper story behind those flashes of light and puffs of smoke. And that narrative is hidden in chemistry jargon, equations, and illustrations. You may also see Solving Equations Worksheet
What triggers a chemical reaction?
There are times when just combining compounds can create a reaction. Sometimes, you need a catalyst or an agent to jumpstart a reaction. These actions can disrupt the bonds between the atoms of the compounds (reactants) and rearrange molecules to form new compounds (products). You may also see Order Of Operations WorkSheet
What are the different kinds of chemical reactions?
After they have learned the basics of balancing chemical equations, students would need to know the different types of chemical reactions. Knowing these four types help the students solve a balancing problem. In synthesis, you produce a compound by combining two or more reactants (H2 + O would give you water). The second type is decomposition. As the opposite of the first, this describes the reaction wherein a reactant separates into products. You can say that the reaction is of the single displacement kind when an ion replaces the other ion in a compound. It is called double displacement when the ions of the reactants trade places. Displacement reactions are characterized by replacing or exchanging of ions between reactants. You may also see Income and Expense WorkSheet
Why should both sides of chemical equations be balanced?
Both sides of the equation should be balanced because even when compounds undergo a chemical reaction, they do not lose mass. If we go back to the law of mass conservation, we can read that neither chemical nor physical reaction can create or destroy matter. The reactions just cause matter to take another form. Therefore, as you go from the left to the right side of the chemical equation, whatever is on the left has to be accounted for on the right side. If you lack one or two elements, you have to find them and balance the equation. You may also see Student Budget WorkSheet
What is the function of the parentheses in chemical equations
Take our photosynthesis example earlier. The product C6H12O6 is a sugar called glucose. When the chemical equation was balanced, this compound appeared as 6(C6H12O6). This means that there are six of the glucose compound. The parentheses are used to preserve the identity of the compound in the equation. If the chemical formula was C12H22O11, this would be known as sucrose. It is still a sugar but it is not glucose. The subscripts show that in sucrose, there are 12 carbon, 22 hydrogen, and 11 oxygen atoms. This is the identity of the compound. If you were to mean that there are 10 glucose products, you place the number 10 outside the parentheses. You may also see Cash Flow WorkSheet
What does the triangle stand for in chemical equations?
The triangle placed on top of the arrow in a chemical equation indicates that heat was introduced into the reaction.
How do you identify reactants and products in the Balancing Chemical Equations Worksheet?
Identifying reactants and products in a chemical equation is crucial for balancing equations accurately.
Steps to Identify Reactants and Products
- Examine the Equation: Look at the chemical equation to determine the substances involved.
- Reactants: These are the substances on the left side of the equation.
- Products: These are the substances on the right side of the equation.
- Use Arrows: The arrow (→) in the equation separates reactants from products.
- Example: In H2+O2→H2OH2+O2→H2O, H2H2 and O2O2 are reactants, and H2OH2O is the product.
- Label Each Substance: Clearly label each chemical formula as a reactant or product.
- Write “R” for reactants and “P” for products.
- Verify States: Check the states of matter (solid, liquid, gas, aqueous) if provided.
- Example: Na(𝑠)+Cl2(𝑔)→NaCl(𝑠)Na(s)+Cl2(g)→NaCl(s)
- Cross-Check with Reactions: Ensure the substances make sense in the context of the reaction.
- Use known chemical reactions as references. You may also see Expense WorkSheet
Why is it important to balance equations in the Balancing Chemical Equations Worksheet?
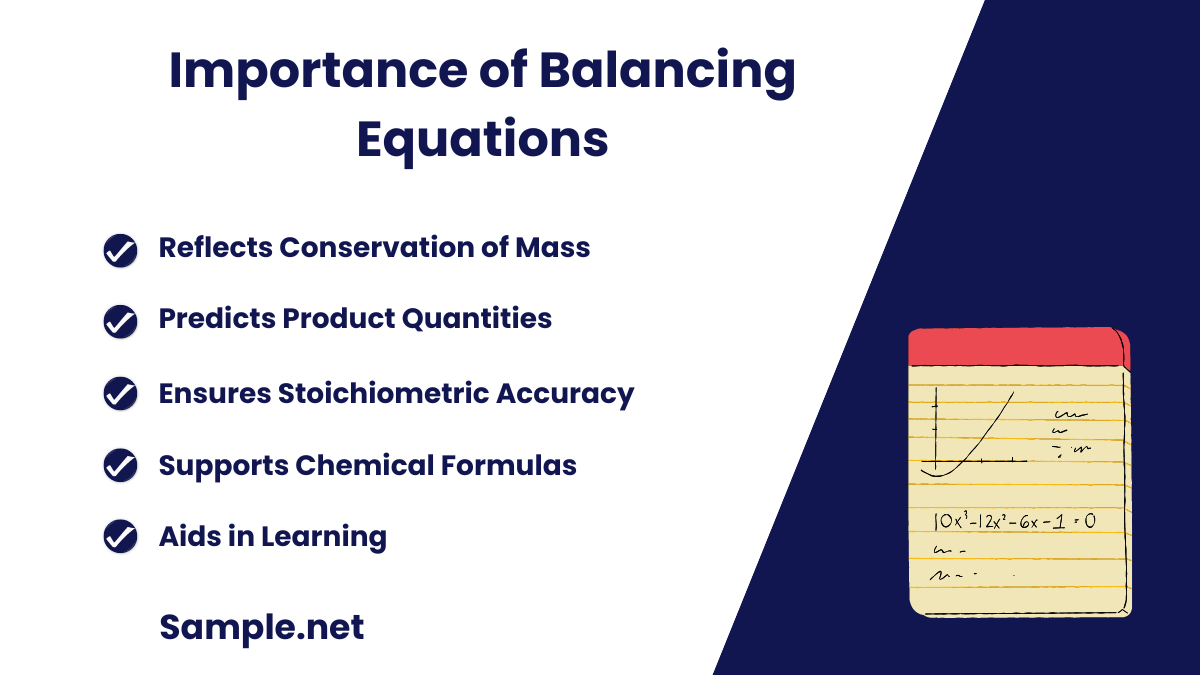
Balancing chemical equations is fundamental to accurately representing chemical reactions and adhering to the law of conservation of mass.
Importance of Balancing Equations
- Reflects Conservation of Mass: Ensures the same number of atoms of each element on both sides.
- Prevents the creation or destruction of matter.
- Predicts Product Quantities: Accurate balancing allows for the prediction of the amounts of products formed.
- Essential for practical applications in chemistry.
- Ensures Stoichiometric Accuracy: Balancing provides correct stoichiometric relationships.
- Crucial for calculations in chemical reactions.
- Supports Chemical Formulas: Validates the chemical formulas of reactants and products.
- Helps in verifying correct chemical structures.
- Aids in Learning: Fundamental for understanding more complex chemical principles.
- Important in academic settings, such as in a Book Report & Reading Worksheet.
How do coefficients help in the Balancing Chemical Equations Worksheet?
Coefficients are numerical values placed in front of chemical formulas to balance equations, ensuring the same number of each type of atom on both sides.
Role of Coefficients
- Balances Atom Counts: Adjusts the number of molecules to equalize atom counts.
- Example: 2H2+O2→2H2O2H2+O2→2H2O
- Maintains Chemical Identity: Keeps chemical formulas unchanged while balancing the equation.
- Coefficients only multiply, not alter, the formulas.
- Ensures Proportions: Reflects the correct proportions of reactants and products.
- Essential for chemical reaction accuracy.
- Facilitates Calculations: Makes it easier to perform stoichiometric calculations.
- Important for practical applications like in a Monthly Budget Worksheet.
- Illustrates Reaction Scale: Shows the scale of the reaction, indicating how much of each substance is involved.
- Helpful in scaling reactions for industrial applications.
What is the role of the conservation of mass in the Balancing Chemical Equations Worksheet?
The conservation of mass states that mass cannot be created or destroyed in a chemical reaction, making it essential for balancing equations.
Conservation of Mass in Balancing Equations
- Equal Mass: Ensures the total mass of reactants equals the total mass of products.
- Validates the principle of mass conservation.
- Atom Balance: Requires the same number of atoms of each element on both sides.
- Prevents discrepancies in the reaction.
- Chemical Integrity: Maintains the integrity of chemical substances.
- Ensures no atoms are lost or gained arbitrarily.
- Predictive Accuracy: Provides accurate predictions for the amounts of reactants and products.
- Important for practical and theoretical chemistry, similar to a Heuristic Evaluation Worksheet.
- Foundation of Chemistry: Fundamental principle underlying all chemical reactions.
- Essential for a deep understanding of chemistry.
What is a systematic approach to solving the Balancing Chemical Equations Worksheet?
A systematic approach ensures that equations are balanced efficiently and accurately.
Systematic Approach Steps
- Write the Unbalanced Equation: List all reactants and products.
- Example: Fe+O2→Fe2O3Fe+O2→Fe2O3
- Count Atoms: Tally the number of each type of atom on both sides.
- Helps identify imbalances.
- Add Coefficients: Adjust coefficients to balance one element at a time.
- Start with the most complex molecule.
- Recount and Adjust: Recount atoms and adjust coefficients as needed.
- Ensures accuracy throughout the process.
- Verify Balance: Confirm that all elements are balanced.
- Final check to ensure the equation is correct. You may also see Value Proposition Worksheet
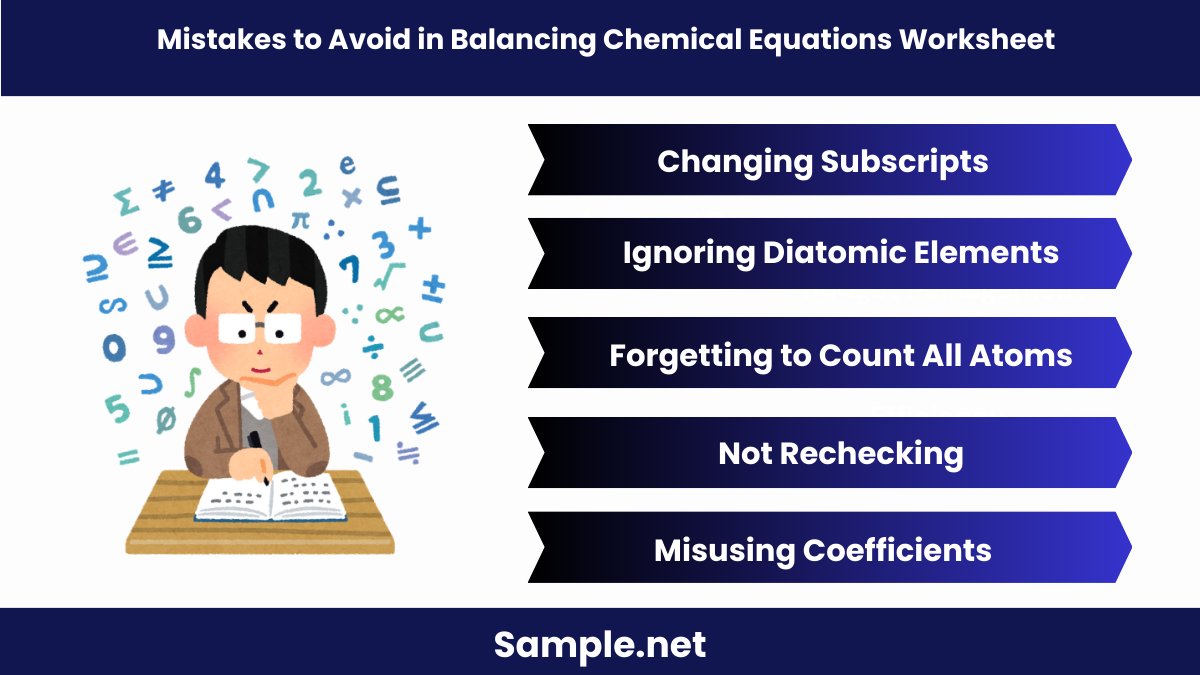
What common mistakes should you avoid in the Balancing Chemical Equations Worksheet?
Avoiding common mistakes ensures accurate and efficient balancing of chemical equations.
Common Mistakes to Avoid
- Changing Subscripts: Never alter subscripts in chemical formulas.
- Changes the substance itself.
- Ignoring Diatomic Elements: Remember that some elements exist as diatomic molecules.
- Example: H2,O2,N2H2,O2,N2
- Forgetting to Count All Atoms: Ensure all atoms, including those in polyatomic ions, are counted.
- Comprehensive counting is crucial.
- Not Rechecking: Always double-check your work.
- Reduces errors in the final equation.
- Misusing Coefficients: Apply coefficients correctly and consistently.
- Incorrect coefficients lead to imbalance. You may also see Printable Pythagorean Theorem Worksheet
What tips are useful for beginners on the Balancing Chemical Equations Worksheet?
Beginners can benefit from specific tips to improve their skills in balancing chemical equations.
Useful Tips for Beginners
- Start Simple: Begin with simple equations and gradually move to complex ones.
- Builds confidence and understanding.
- Practice Regularly: Consistent practice improves proficiency.
- Similar to practicing with a Pythagorean Theorem Worksheet.
- Use Visual Aids: Draw diagrams or use models to visualize the reaction.
- Helps in understanding the balancing process.
- Learn Polyatomic Ions: Familiarize yourself with common polyatomic ions.
- Makes balancing easier when they appear in equations.
- Ask for Help: Don’t hesitate to seek assistance from teachers or peers.
- Collaboration enhances learning, as with a Business Plan Worksheet.
How does stoichiometry help with the Balancing Chemical Equations Worksheet?
Stoichiometry helps balance chemical equations by ensuring the correct proportions of reactants and products. It provides a quantitative framework for understanding chemical reactions, similar to a SWOT Analysis Worksheet in strategic planning.
What is the first step in the Balancing Chemical Equations Worksheet?
The first step in the Balancing Chemical Equations Worksheet is to write the unbalanced equation, listing all reactants and products. This sets the foundation for systematically balancing the equation, much like starting a Cursive Writing Worksheet.
Why is it important to double-check your work on the Balancing Chemical Equations Worksheet?
Double-checking your work ensures accuracy in balancing chemical equations, preventing errors and reinforcing your understanding of reactions. This careful review process is akin to finalizing a Wedding Budget Worksheet for precision.
How do you handle complex equations on the Balancing Chemical Equations Worksheet?
Handle complex equations by balancing one element at a time, starting with the most complex molecule. Break down the process into manageable steps, similar to tackling a Zero Based Budget Worksheet.
How does the Balancing Chemical Equations Worksheet improve your understanding of reactions?
The Balancing Chemical Equations Worksheet improves understanding by reinforcing the law of conservation of mass and illustrating how reactants transform into products. This foundational knowledge is as critical as mastering an Income Worksheet.
How can you verify an equation is balanced on the Balancing Chemical Equations Worksheet?
Verify an equation is balanced by ensuring the number of atoms of each element is equal on both sides. This verification process is essential, much like confirming calculations in a Multiplication Worksheet.
How does practicing with the Balancing Chemical Equations Worksheet enhance problem-solving skills?
Practicing with the Balancing Chemical Equations Worksheet enhances problem-solving skills by developing logical thinking and precision. This practice is similar to using a Goal Setting Worksheet to achieve structured, measurable objectives.
Balancing chemical equations is a crucial skill in understanding chemical reactions. Our article on the Balancing Chemical Equations Worksheet provides sample problems, forms, and detailed explanations to guide learners. Using this worksheet, students can practice and refine their balancing techniques, ensuring they grasp the fundamental concepts of chemistry. This resource is invaluable for both classroom use and individual study, helping students achieve proficiency in solving equations. Utilize our guide to enhance your chemistry skills and confidently tackle any chemical equation.