45+ Balancing Chemical Equations Worksheets (College, Chemistry, Students)
-

8th Grade Balancing Chemical Equations Worksheet with Answer Key
download now -

Chemistry Balancing Chemical Equations Worksheet with Answer Key
download now -

Balancing Chemical Equations Worksheet for Practice
download now -

6th Grade Balancing Chemical Equations Worksheet
download now -

Balancing Chemical Equations Worksheet for Science
download now -

Balancing Act Chemical Equation Worksheet
download now -

Balancing Chemical Equation Worksheet For Homework with Answer Key
download now -

Unit 7 Balancing Chemical Equations Worksheet
download now -

Balancing Chemical Equations Worksheet for Grade 10
download now -

GCSE Balancing Chemical Equations Worksheet
download now -

Balancing Chemical Equations Worksheet for Students
download now -

Balancing Chemical Equations Worksheet and Type of Reaction
download now -

Fair Balancing Chemical Equation Worksheet
download now -
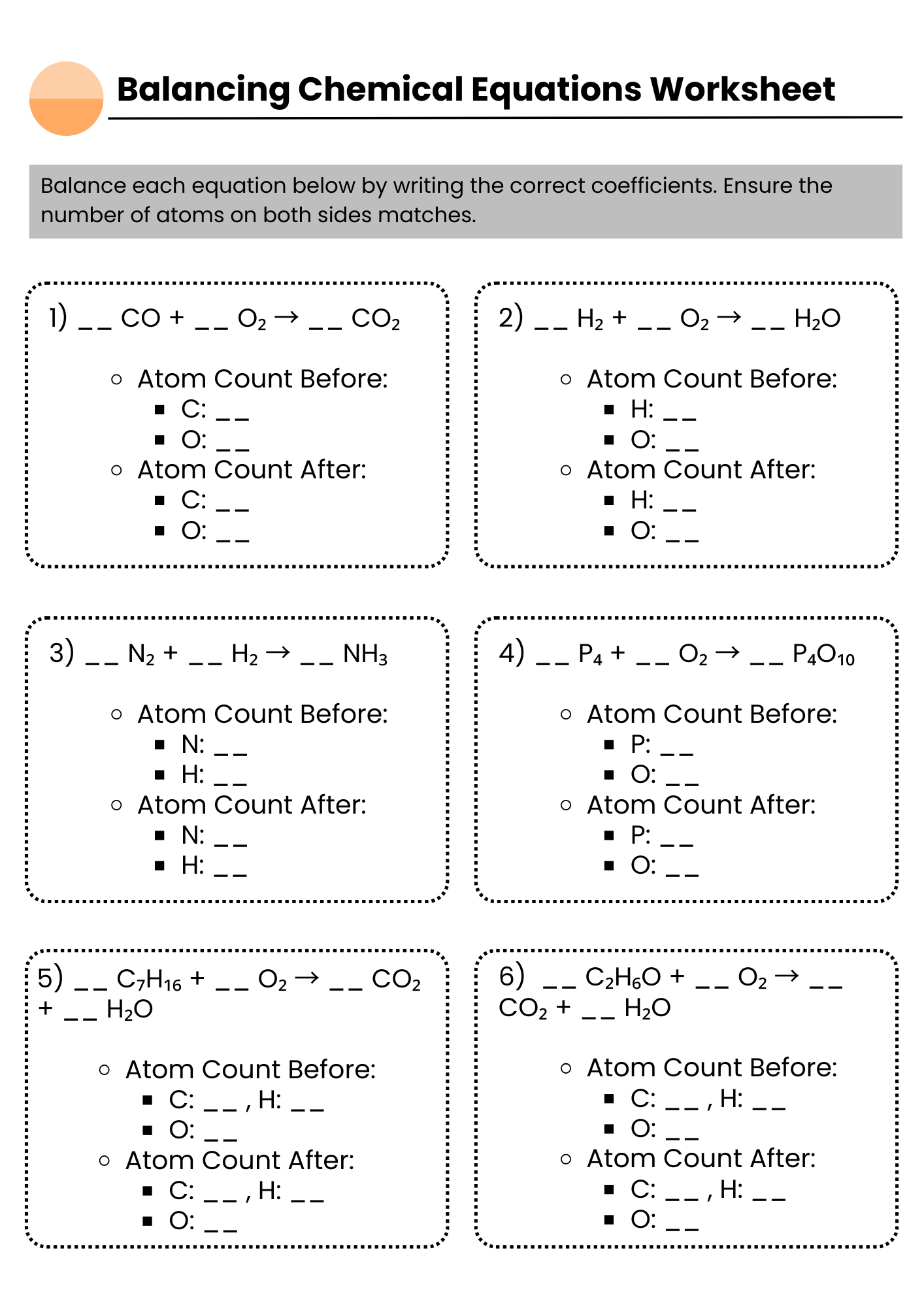
Balancing Chemical Equations Worksheet Counting Chart
download now -

NaI Balancing Chemical Equations Worksheet
download now -
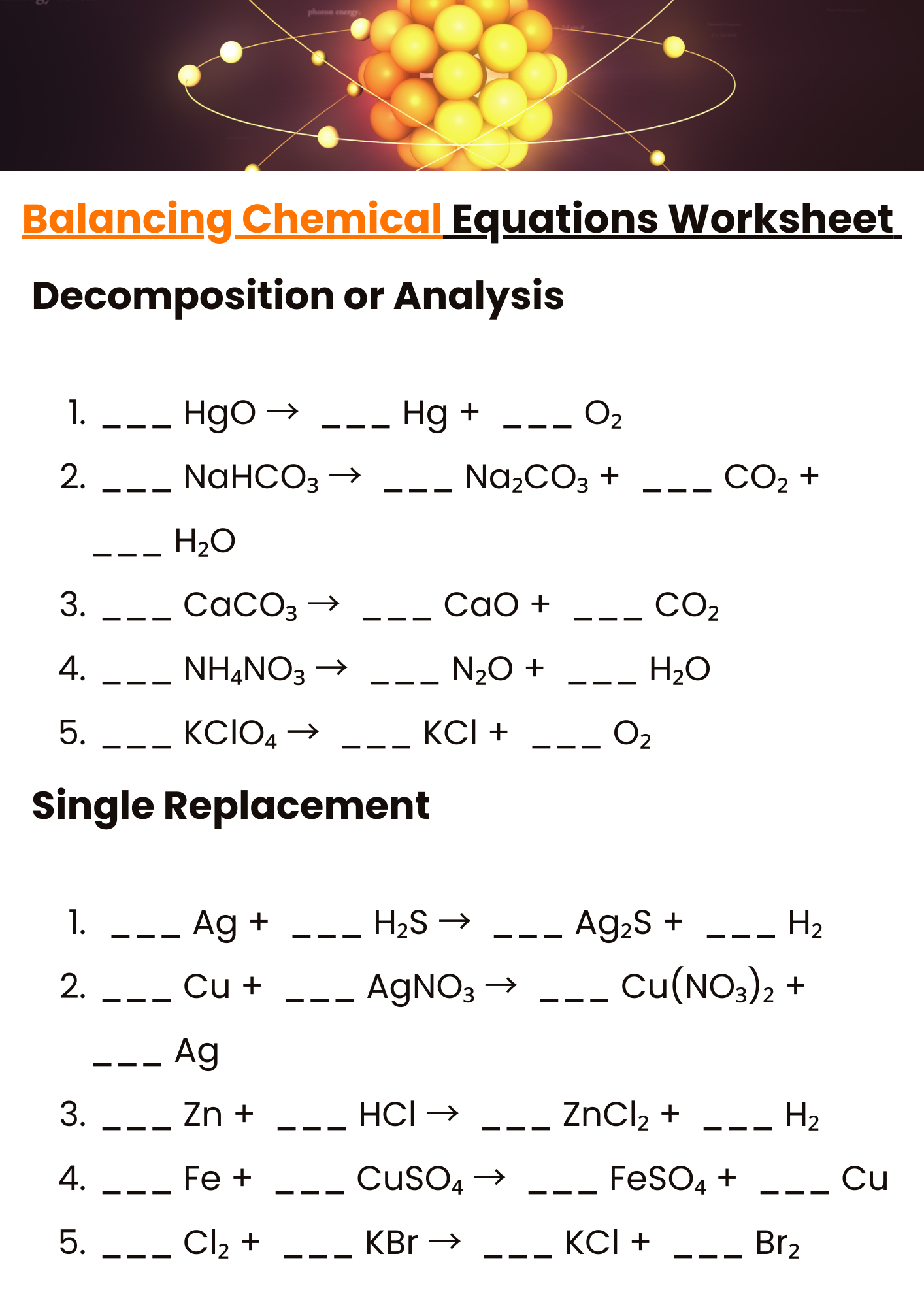
Practicing Balancing Chemical Equations Worksheet
download now -

Balancing Chemical Equations Worksheet for Beginners
download now -

Balancing Chemical Equations Worksheet for Class 10
download now -

Balancing Chemical Equations Worksheet for Middle School
download now -

Balancing Chemical Equations Worksheet for Class 9
download now -

Balancing Chemical Equations Worksheet for Class 8
download now -
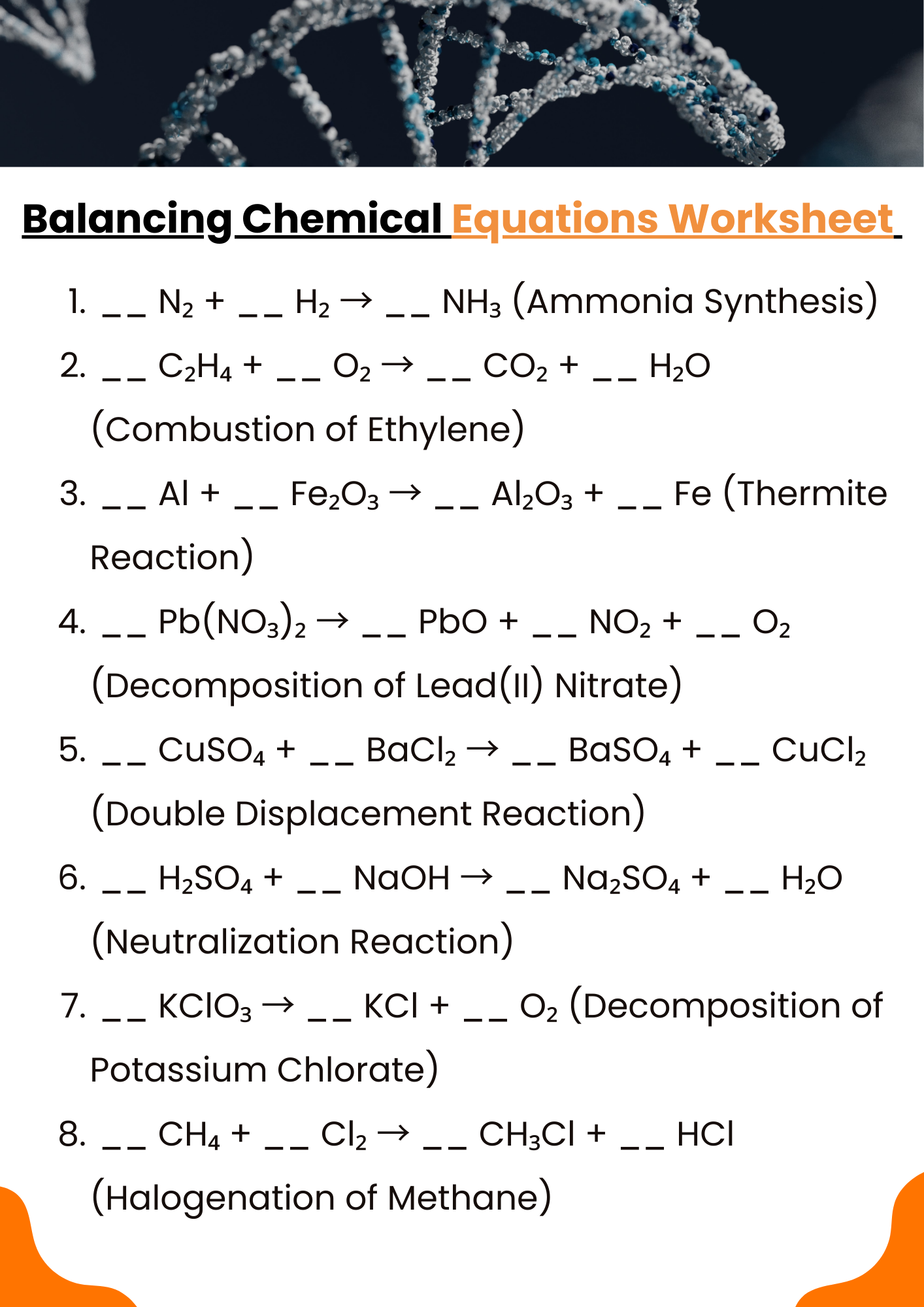
10th Grade Balancing Chemical Equations Worksheet
download now -

9th Grade Balancing Chemical Equations Worksheet
download now -

Redox Balancing Chemical Equations Worksheet
download now -
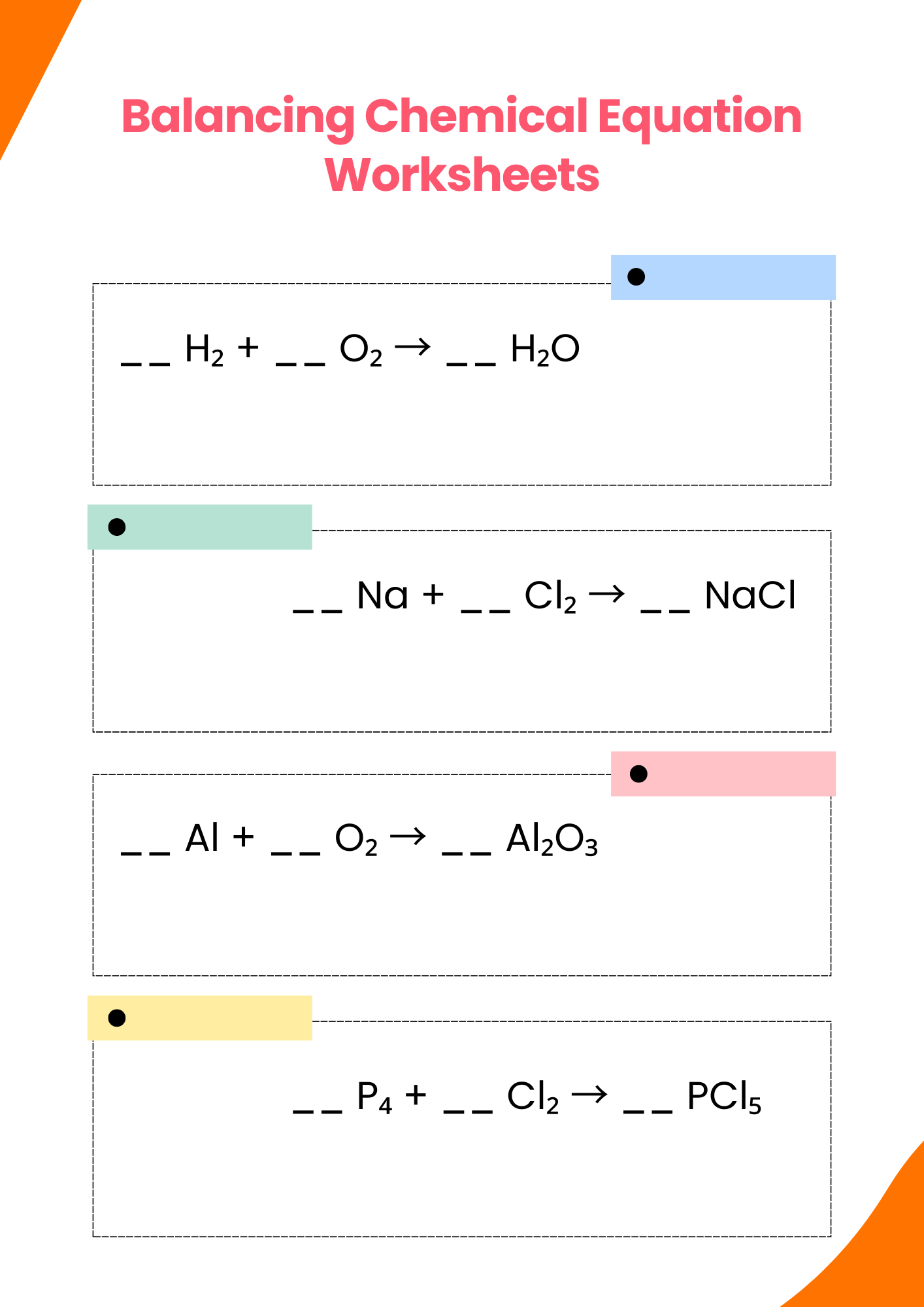
PHET Balancing Chemical Equation Worksheet
download now -

Blank Balancing Chemical Equations Worksheet
download now -

Classifying & Balancing Chemical Equations Worksheet
download now -
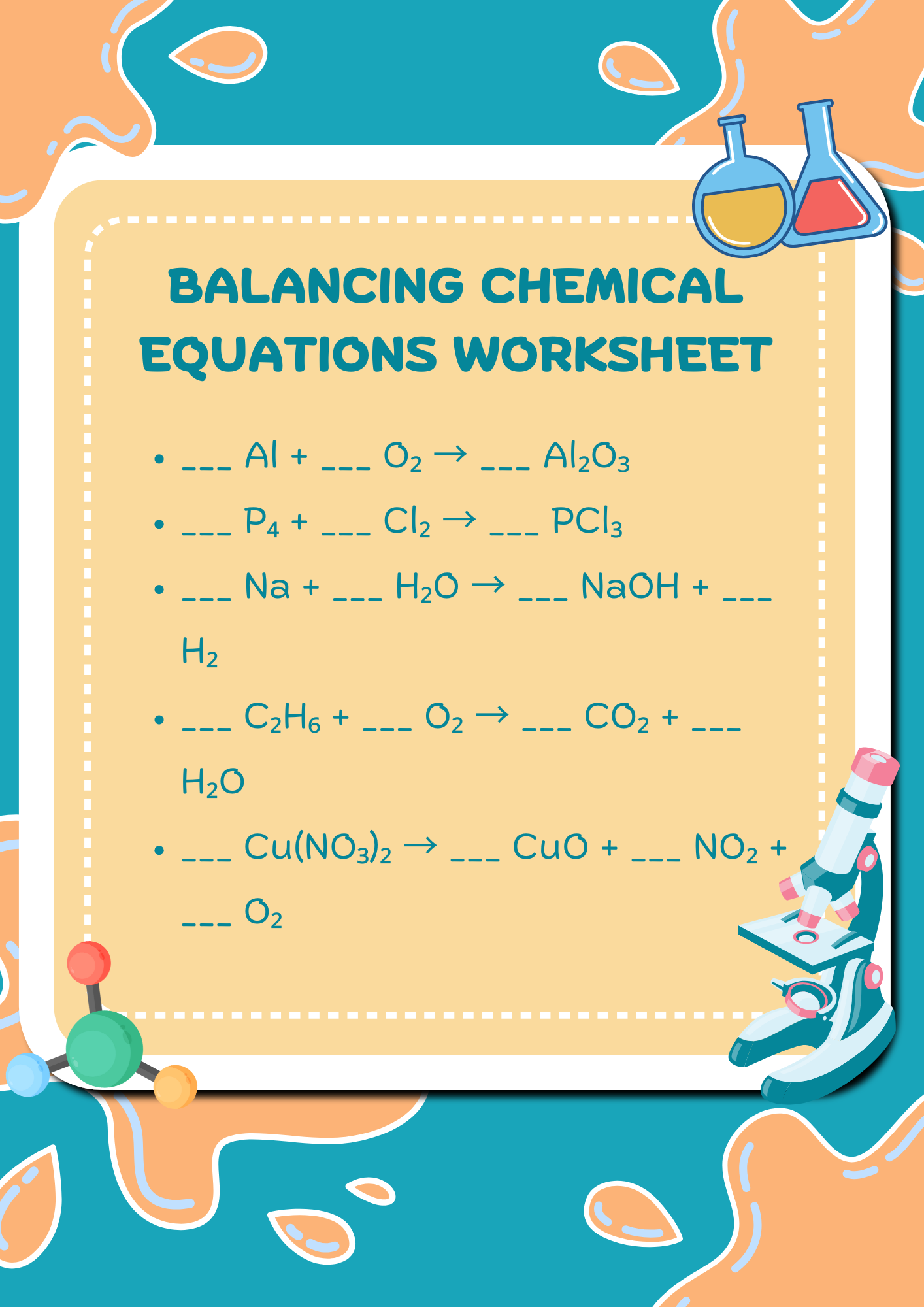
Sciencenotes Balancing Chemical Equations Worksheet
download now -

Balancing Chemical Equations Worksheet for Test Preparation
download now -

Theoretical Balancing Chemical Equations Worksheet
download now -

Balancing Chemical Equation Worksheet for Acid – Base Reactions
download now -

Balancing Chemical Equation Worksheet and Reactions
download now -

Printable Balancing Chemical Equation Worksheet
download now -

Interactive Balancing Chemical Equation Worksheet
download now -
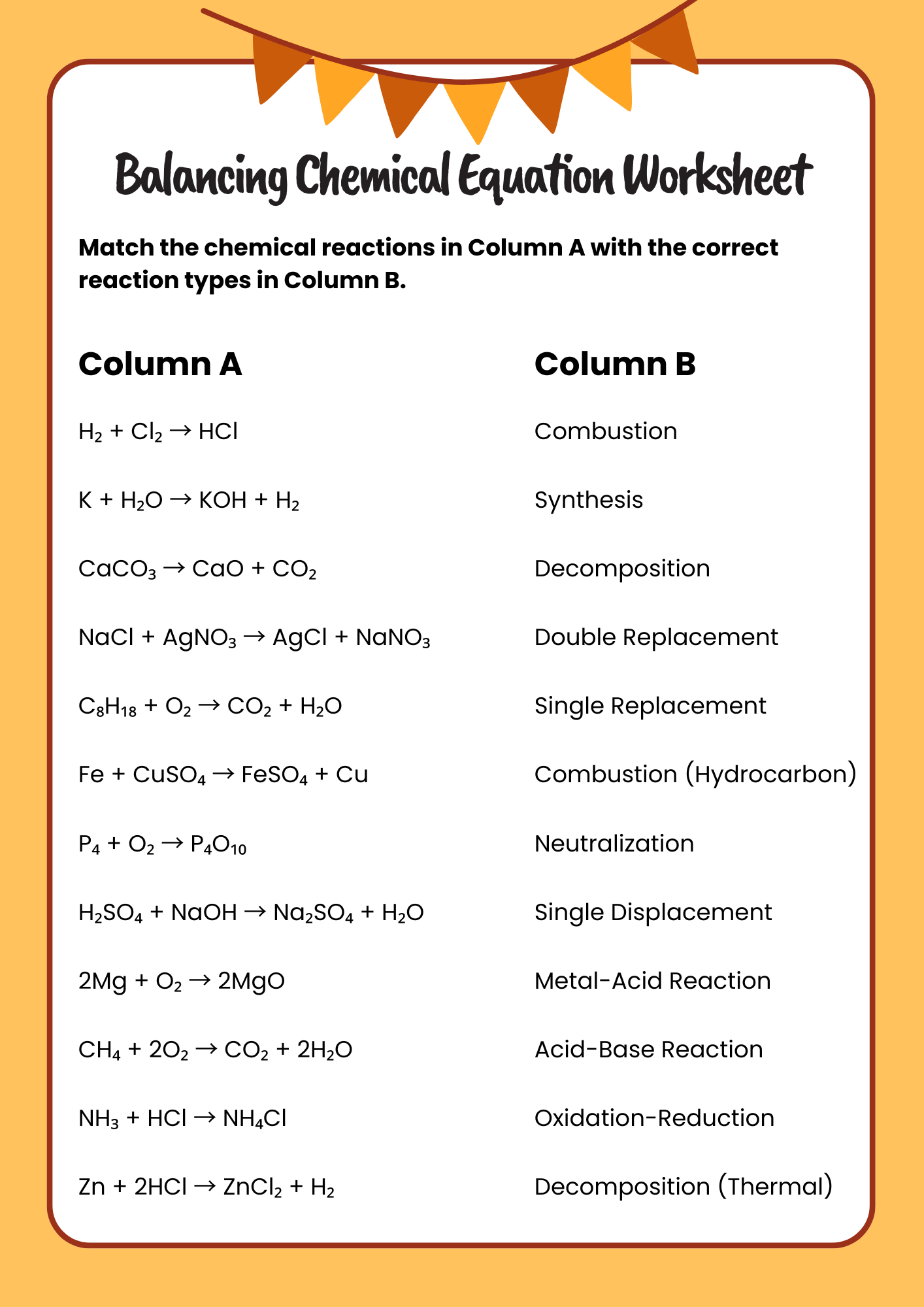
Elementary Balancing Chemical Equation Worksheet
download now -

Advance Balancing Chemical Equation Worksheet
download now -
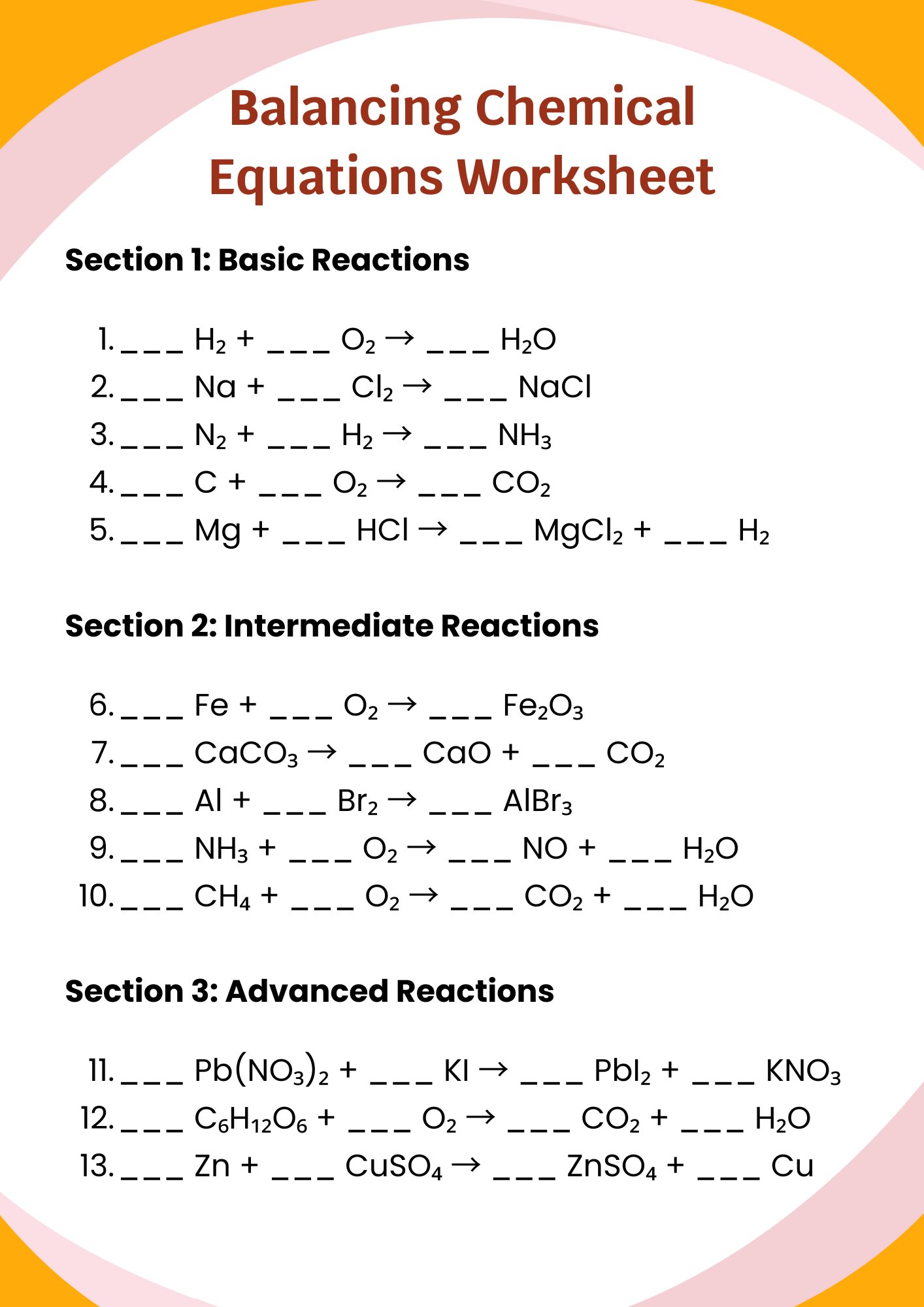
Balancing Chemical Equations Worksheet for Teacher
download now -

Practicing Balancing Chemical Equations Worksheet
download now -

Balancing Chemical Equations Worksheet for Classroom
download now -

Simple Balancing Chemical Equations Worksheet with Answer Key
download now -
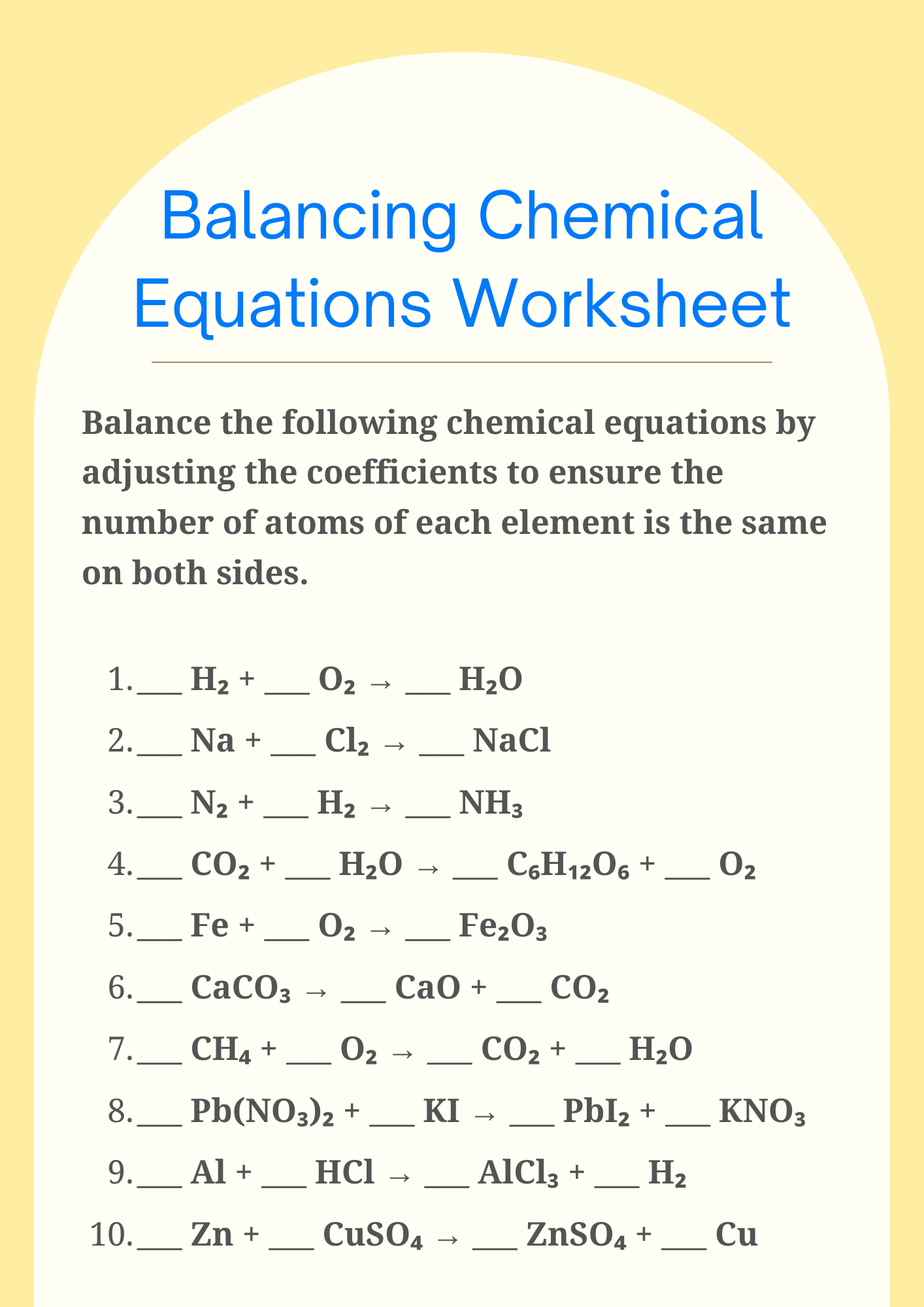
Balancing Chemical Equations Worksheet for Preparation
download now -

Balancing Chemical Equation Worksheet for Class 7
download now -

5th Grade Balancing Chemical Equations Worksheet
download now -

Stereochemistry Balancing Chemical Equations Worksheet
download now -

Writing and Balancing Chemical Equations Worksheet
download now -

Balancing Chemical Equations Worksheet for High School
download now -

Conceptual Balancing Chemical Equations Worksheet
download now
Free Printable Balancing Chemical Equations Worksheet s to Download
45+ Balancing Chemical Equations Worksheets (College, Chemistry, Students)
What is a Balancing Chemical Equations?
How to Create Balancing Chemical Equations Worksheet
Steps for Balancing Chemical Equations
How to Use a Balancing Chemical Equations Worksheet?
Why a Balancing Chemical Equations Worksheet Important?
Tips for Balancing Chemical Equations
What is a Balancing Chemical Equations?
Balancing chemical equations is a process used in chemistry to ensure that the number of atoms for each element is the same on both sides of a chemical equation. This is important because it reflects the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. When you balance a chemical equation, you adjust the numbers in front of the chemical formulas (these numbers are called coefficients) until the amount of atoms of each element is equal on both sides. This process makes sure the equation is accurate and obeys the natural laws of chemistry.
How to Create Balancing Chemical Equations Worksheet

1️⃣ Decide the Target Audience
Choose whether the worksheet is for middle school, high school, or college students.
2️⃣ Select Reaction Types
Include a mix of reactions: synthesis, decomposition, single replacement, double replacement, and combustion.
3️⃣ Gather Unbalanced Equations
Pick or create 8–15 unbalanced chemical equations that match the students’ skill level.
4️⃣ Design Clear Layout
Format the worksheet with enough space for students to write coefficients next to each equation.
5️⃣ Add Word Problems (Optional)
Include a few word equations for students to translate into balanced chemical equations.
6️⃣ Add a Reaction Type Column
Let students identify the type of chemical reaction after balancing.
7️⃣ Prepare an Answer Key
Create a separate solution page for checking or teacher use.
8️⃣ Save and Format for Sharing
Save the worksheet as a PDF or DOC file for easy printing or digital distribution.
Steps for Balancing Chemical Equations
✔️ Identify the Reactants and Products: Start by writing down the correct chemical formulas for the reactants on the left side and the products on the right side of the equation.
✔️ List the Number of Atoms: Write down the number of atoms of each element present in both the reactants and products to see which elements need balancing.
✔️ Choose One Element to Start: Begin with an element that appears in only one reactant and one product, if possible. This simplifies the initial balancing.
✔️ Adjust Coefficients, Not Subscripts: Change the numbers before the chemical formulas (coefficients) to balance the atoms of the selected element. Never change the subscripts within a chemical formula.
✔️ Balance Remaining Elements: Move on to balance other elements in the equation, adjusting their coefficients as needed. Save elements like hydrogen and oxygen for last, as they often appear in multiple compounds.
✔️ Check the Balance of Each Element: After adjusting, make sure each element has the same number of atoms on both sides of the equation.
✔️ Repeat if Necessary: If the equation is not balanced, go through the steps again and adjust the coefficients until the equation is balanced.
✔️ Verify the Charges: If you are balancing a net ionic equation, ensure that the total charges on both sides are equal.
✔️ Simplify the Coefficients: If possible, simplify the coefficients to their lowest whole numbers that balance the equation.
✔️ Review and Finalize: Double-check your balanced equation to ensure accuracy. Confirm that both mass and charge are conserved.
How to Use a Balancing Chemical Equations Worksheet?

1️⃣ Review Basic Concepts: Before starting, ensure that you understand the basic concepts of chemical equations, including reactants, products, and the law of conservation of mass.
2️⃣ Read the Instructions Carefully: Each worksheet may have specific instructions or guidelines. Make sure to read these instructions to understand what is expected in terms of balancing the equations.
3️⃣ Identify the Elements: For each equation, list all the elements involved in the reactants and products. This will help you track each atom throughout the balancing process.
4️⃣ Count the Atoms of Each Element: Before balancing, count the number of atoms of each element on both sides of the equation to see which are unbalanced.
5️⃣ Adjust Coefficients, Not Subscripts: Use coefficients to balance the equations. Remember not to change the subscripts of the compounds, as this would alter the substances involved.
6️⃣ Check Your Work: After balancing the equations, recheck the atom counts to ensure that both sides are balanced. This verifies adherence to the conservation of mass.
7️⃣ Practice Regularly: Use the worksheet to practice regularly. Balancing chemical equations is a skill that improves with practice.
8️⃣ Seek Feedback: If possible, have a teacher or a knowledgeable peer review your balanced equations to provide feedback and help correct any mistakes.
Why a Balancing Chemical Equations Worksheet Important?
✅ Reinforces the Law of Conservation of Mass: It helps students understand that mass cannot be created or destroyed in a chemical reaction, which is a fundamental concept in chemistry.
✅ Develops Problem-Solving Skills: Balancing equations challenges students to apply logic and mathematical skills, enhancing their ability to solve complex problems.
✅ Prepares for Advanced Chemistry: Mastery of balancing chemical equations is crucial for success in more advanced chemistry topics and laboratory work.
✅ Enhances Understanding of Chemical Reactions: Students gain insights into the reactants and products involved in chemical reactions, including their stoichiometric relationships.
✅ Improves Attention to Detail: The process requires careful attention to the types and quantities of atoms involved, fostering meticulousness necessary for scientific studies.
Tips for Balancing Chemical Equations
-
Write the Correct Formulas: Ensure you have the correct chemical formulas for all reactants and products before starting.
-
Count the Atoms: Tally the number of atoms for each element on both sides of the equation to see what needs balancing.
-
Start with Complicated Molecules: Begin balancing with the compound that contains the most elements or largest subscripts.
-
Balance Metals First: If metals and non-metals are involved, start by balancing the metals as they typically appear less frequently.
-
Use Coefficients Wisely: Adjust the coefficients in front of compounds to make the number of atoms equal on both sides.
-
Balance Hydrogens and Oxygens Last: Since hydrogen and oxygen are common, balance them after all other elements have been adjusted.
-
Double-check Your Work: After balancing, verify that each element has an equal number of atoms on both sides of the equation.
-
Use a Pencil: When working on paper, use a pencil to easily make corrections as you find the balance.
-
Practice Regularly: Regular practice with a variety of equations will improve your balancing skills.
-
Seek Help If Needed: Don’t hesitate to ask for help from teachers or peers if you’re struggling to understand or balance equations. Sometimes a fresh pair of eyes can offer a new perspective or solution.
