50+ Counting Atoms Worksheets (Chemistry, High School, Atoms, Element, Compound, Ionic, Molecule, 7th Grade, 8th Grade, 9th Grade, 10th Grade)
-
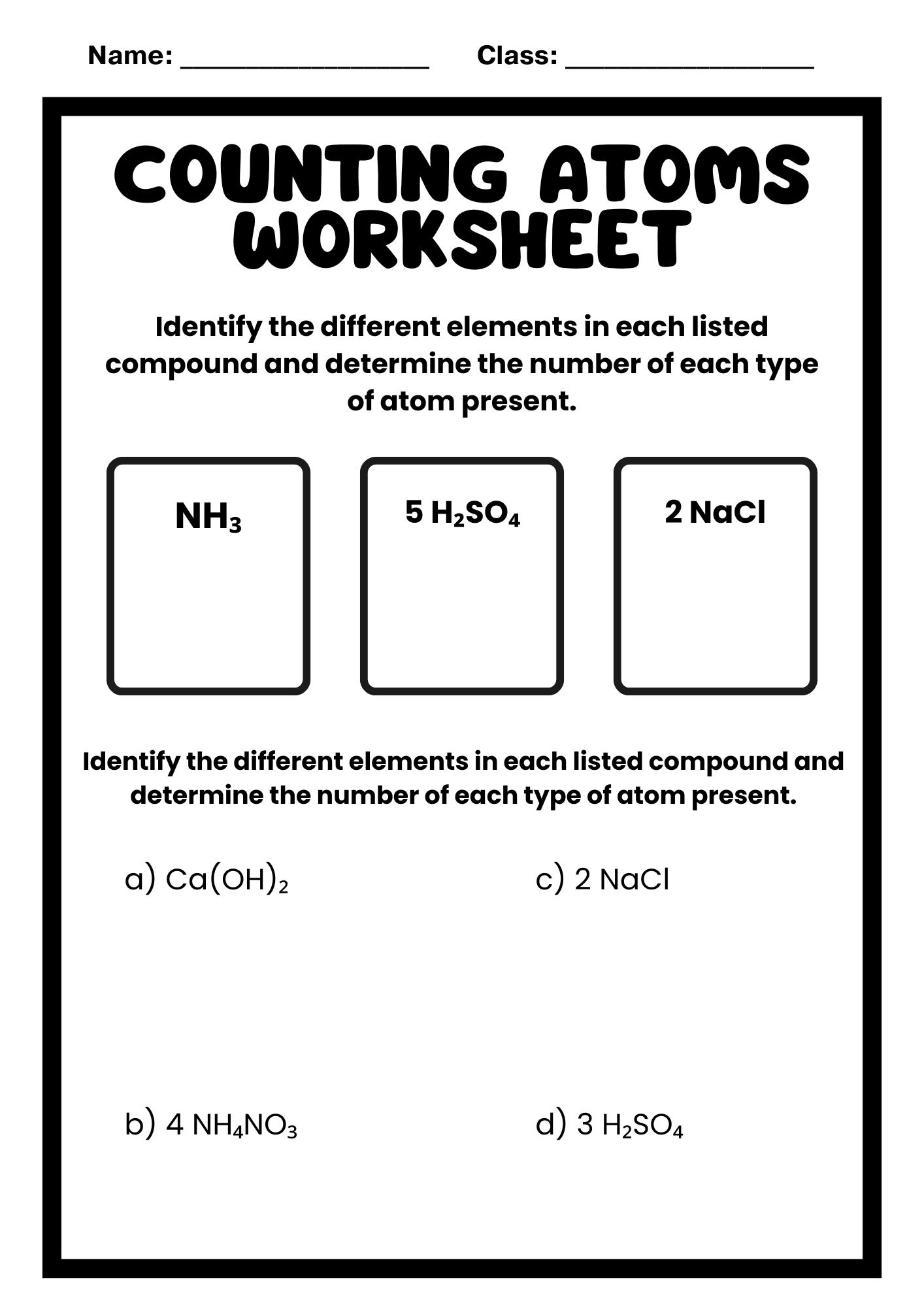
Counting Atoms Worksheet for College Students
download now -
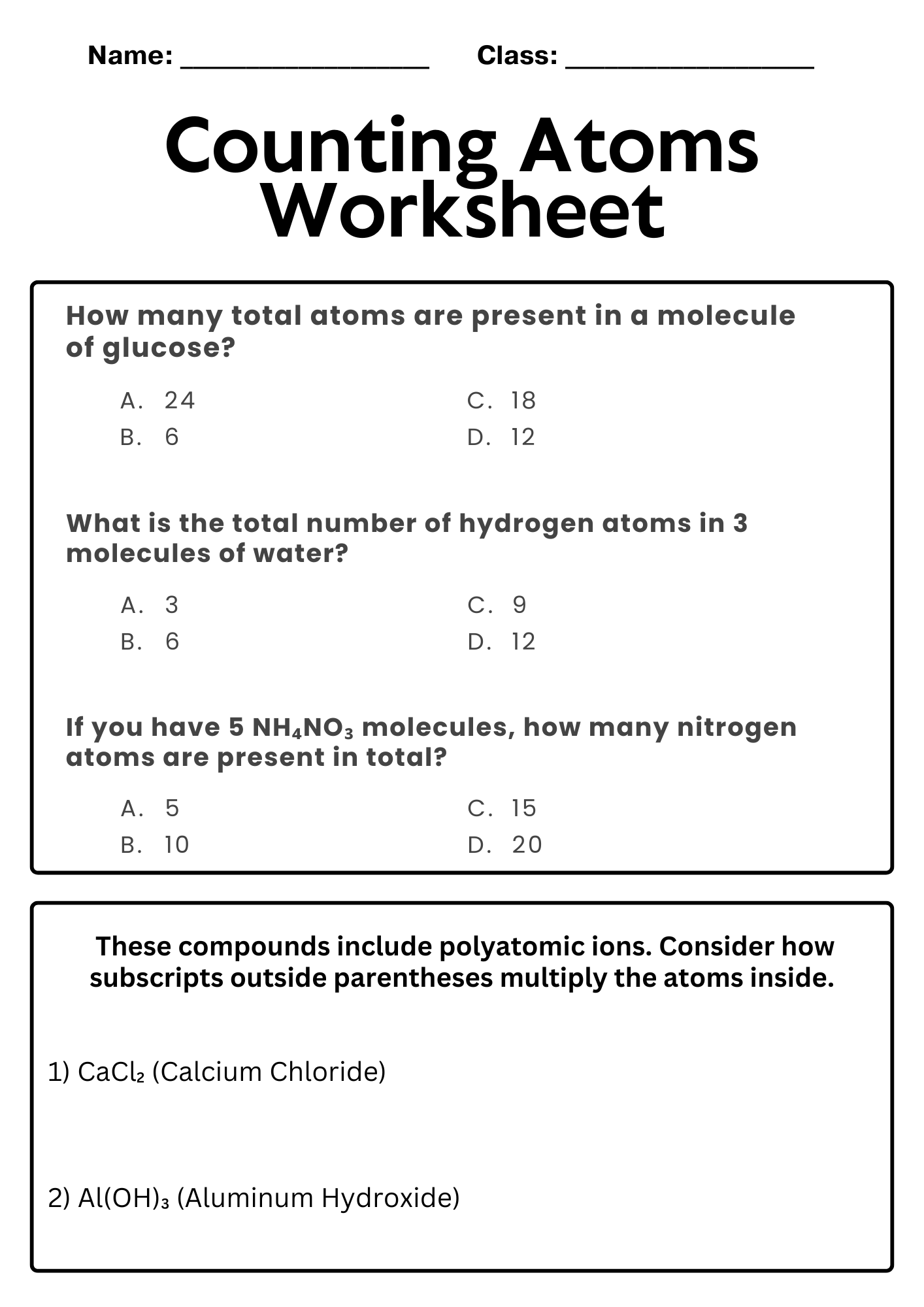
Counting Atoms Worksheet for Quiz
download now -
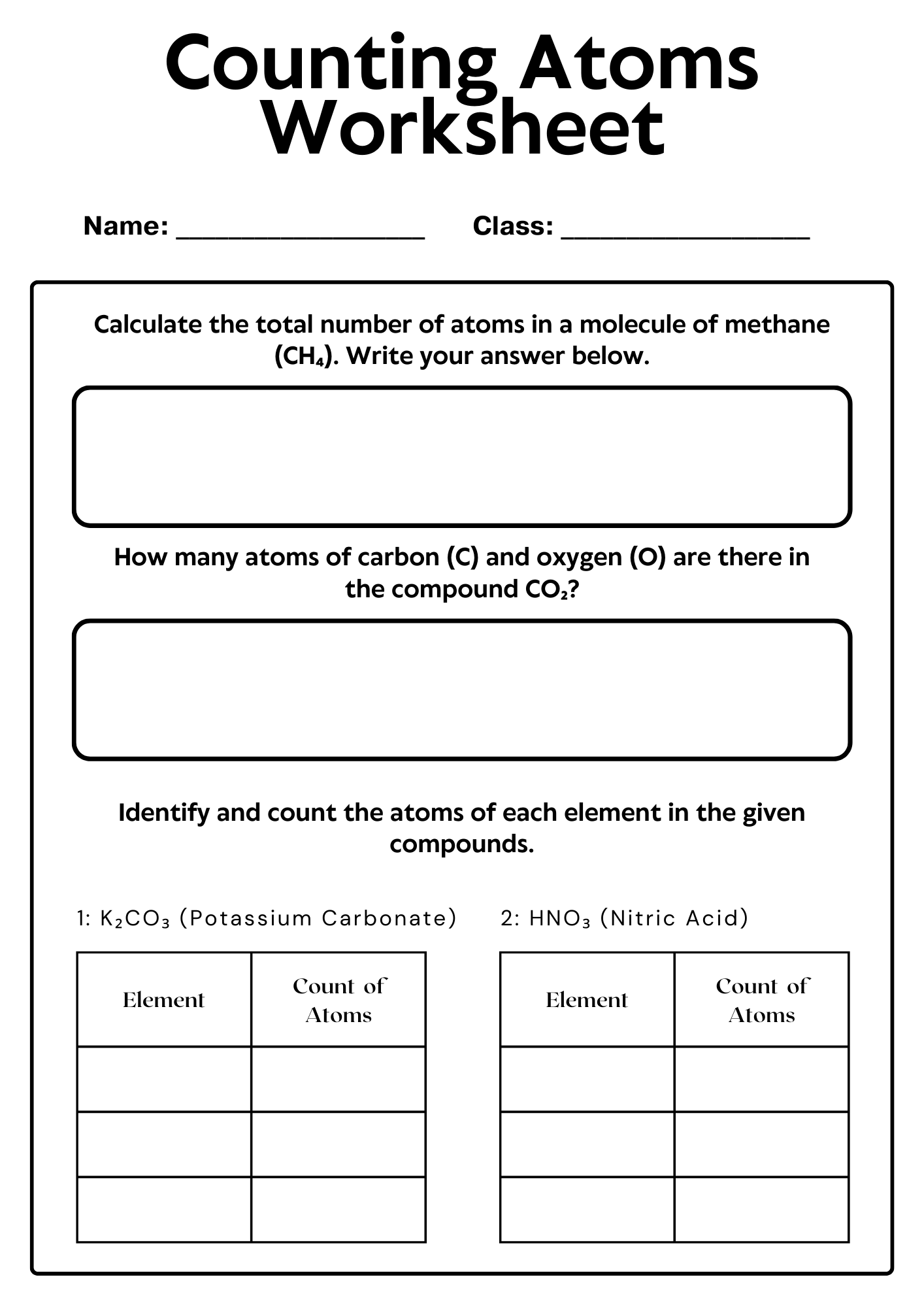
Counting Atoms Worksheet for Exam Preparation
download now -
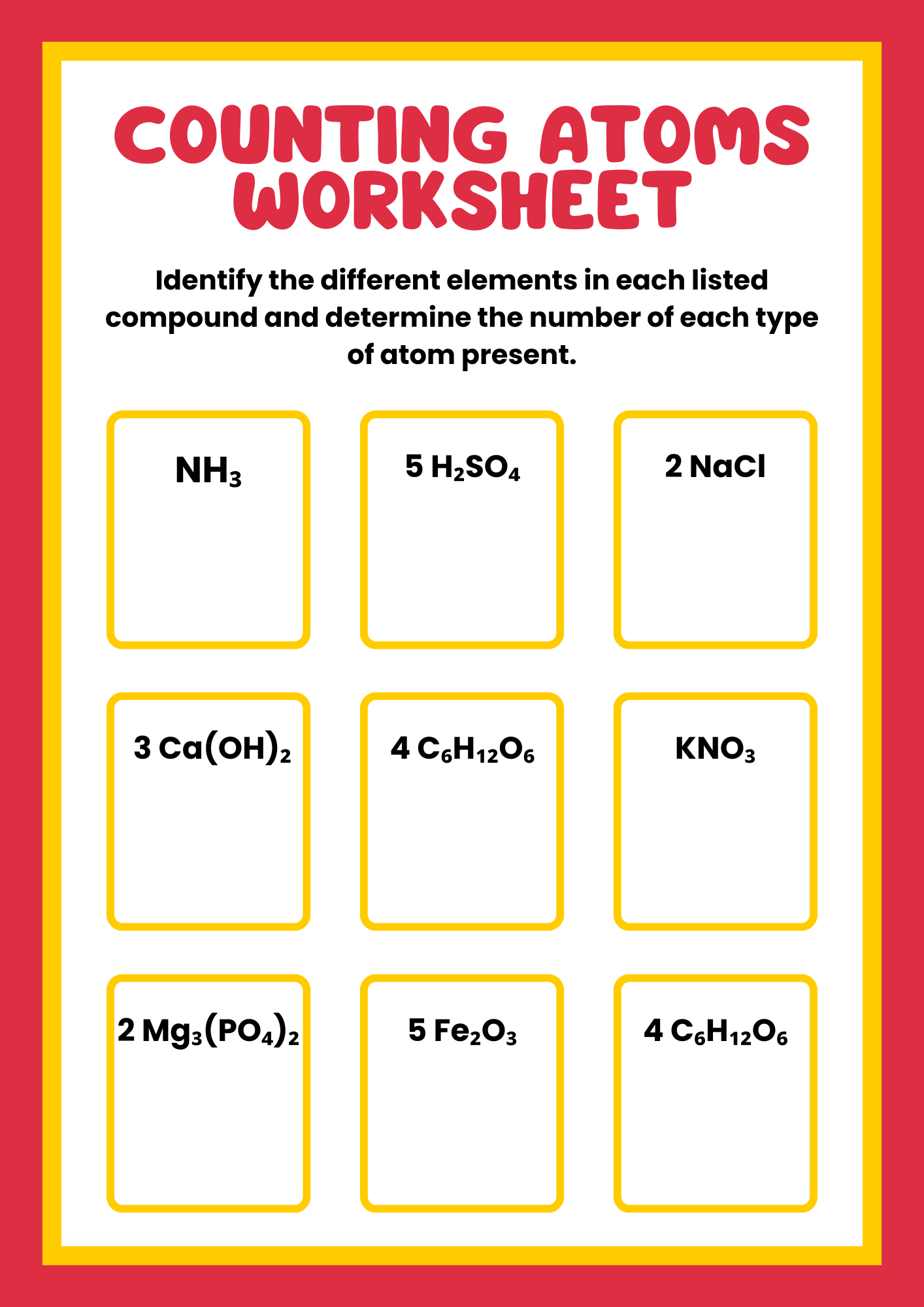
Simple Counting Atoms Worksheet
download now -
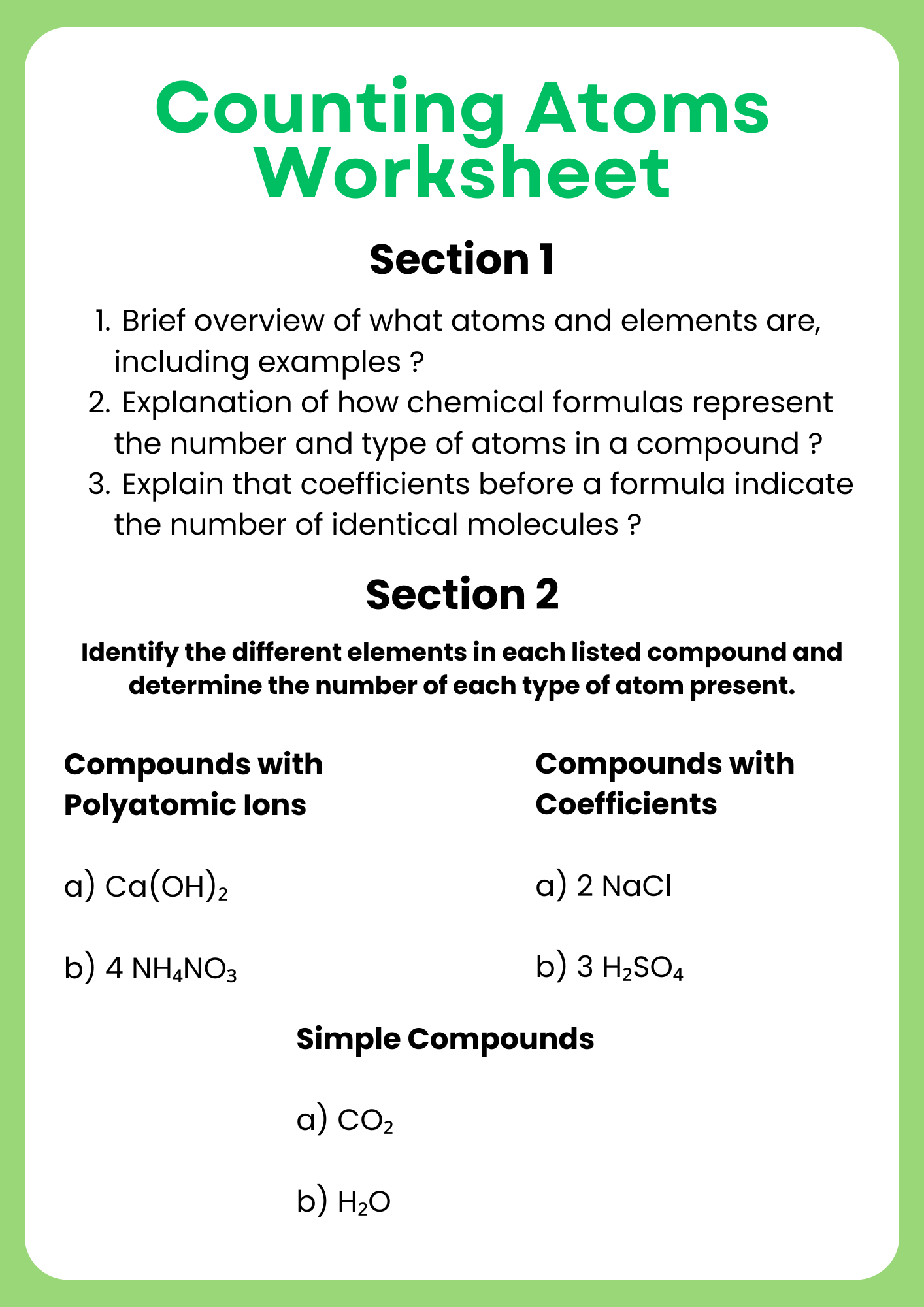
Counting Atoms Worksheet for Chemistry
download now -
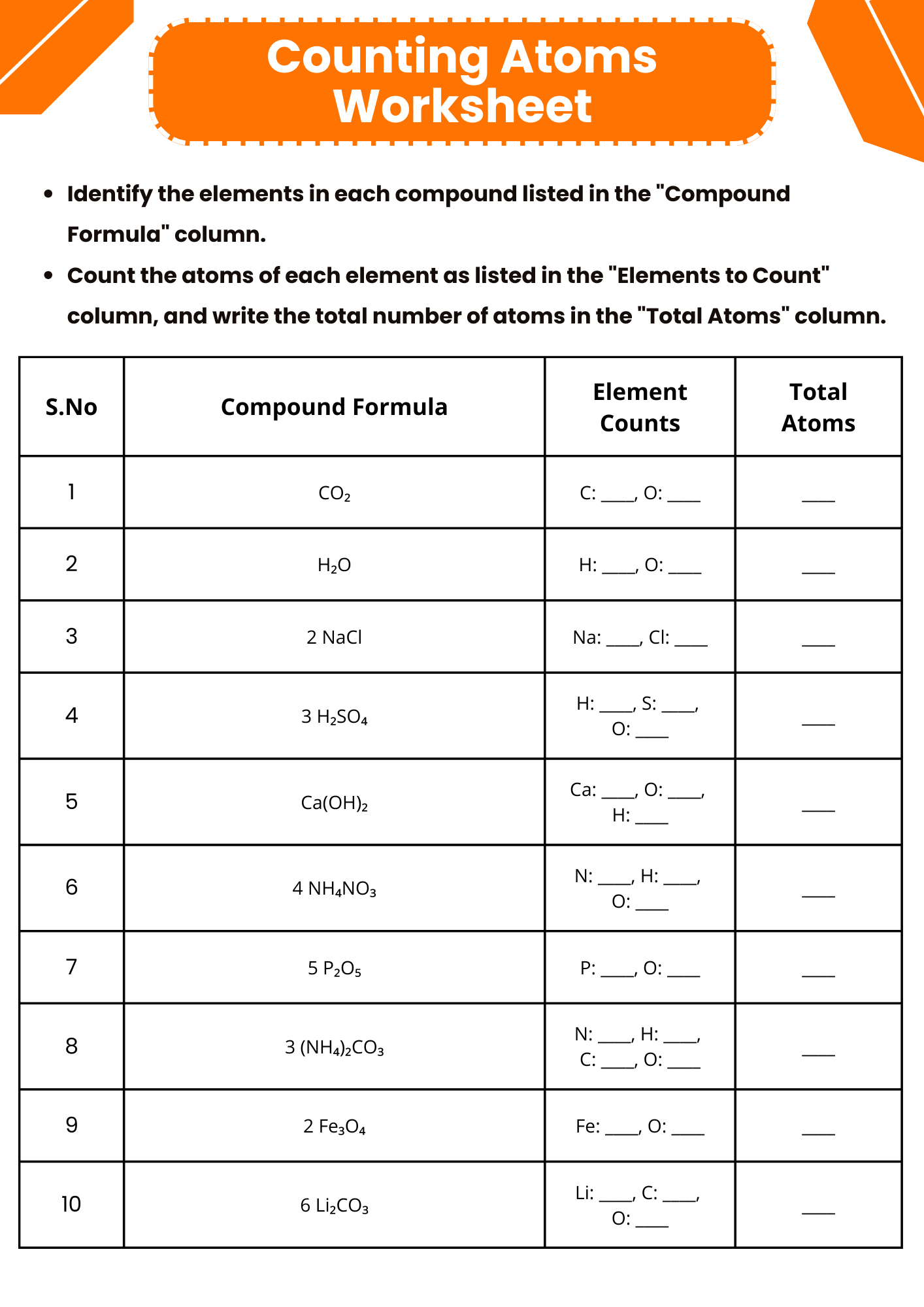
Printable Counting Atoms Worksheet
download now -
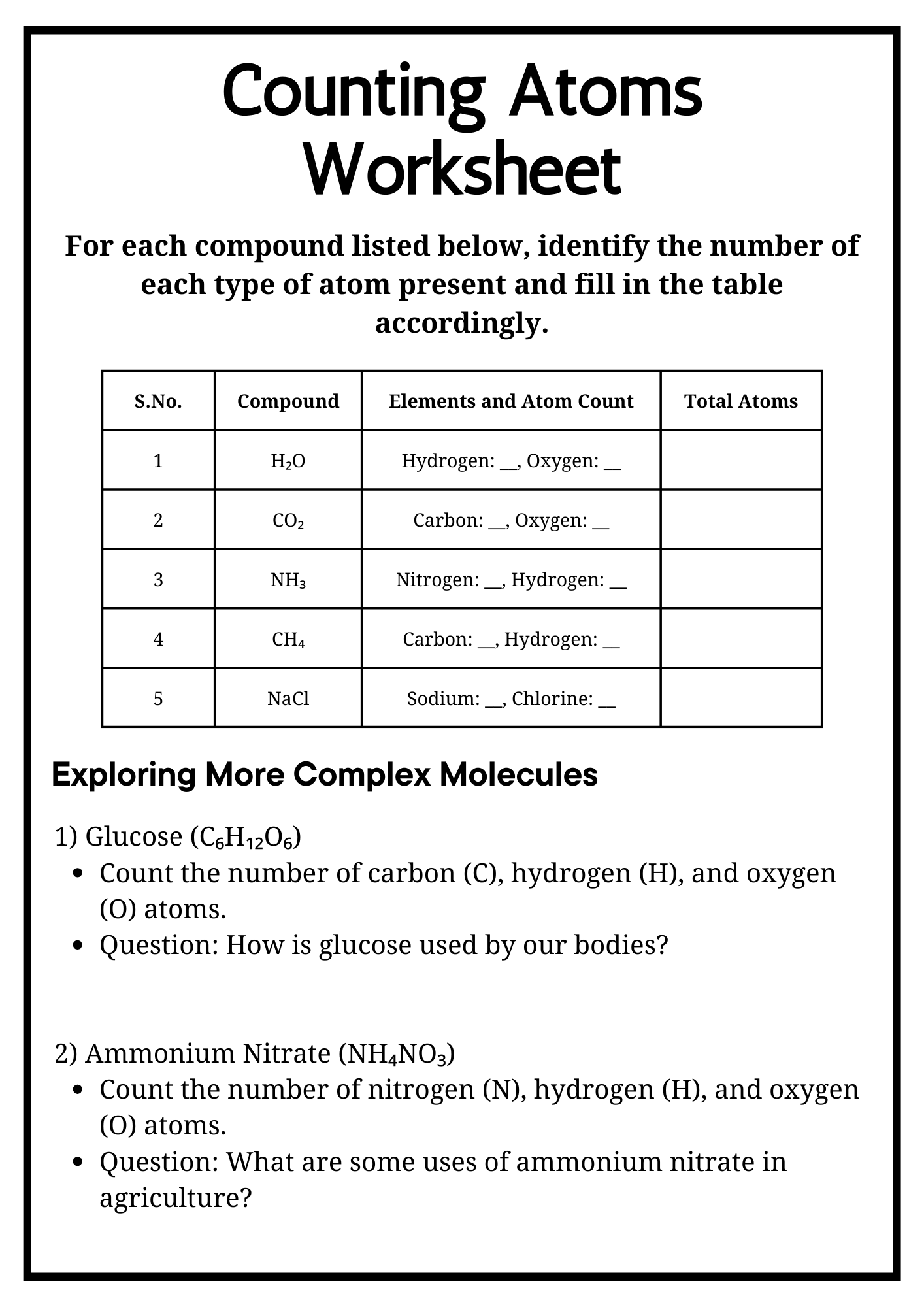
Counting Atoms Worksheet for Test
download now -

Counting Atoms Worksheet for High School
download now -

Easy Counting Atoms Worksheets
download now -
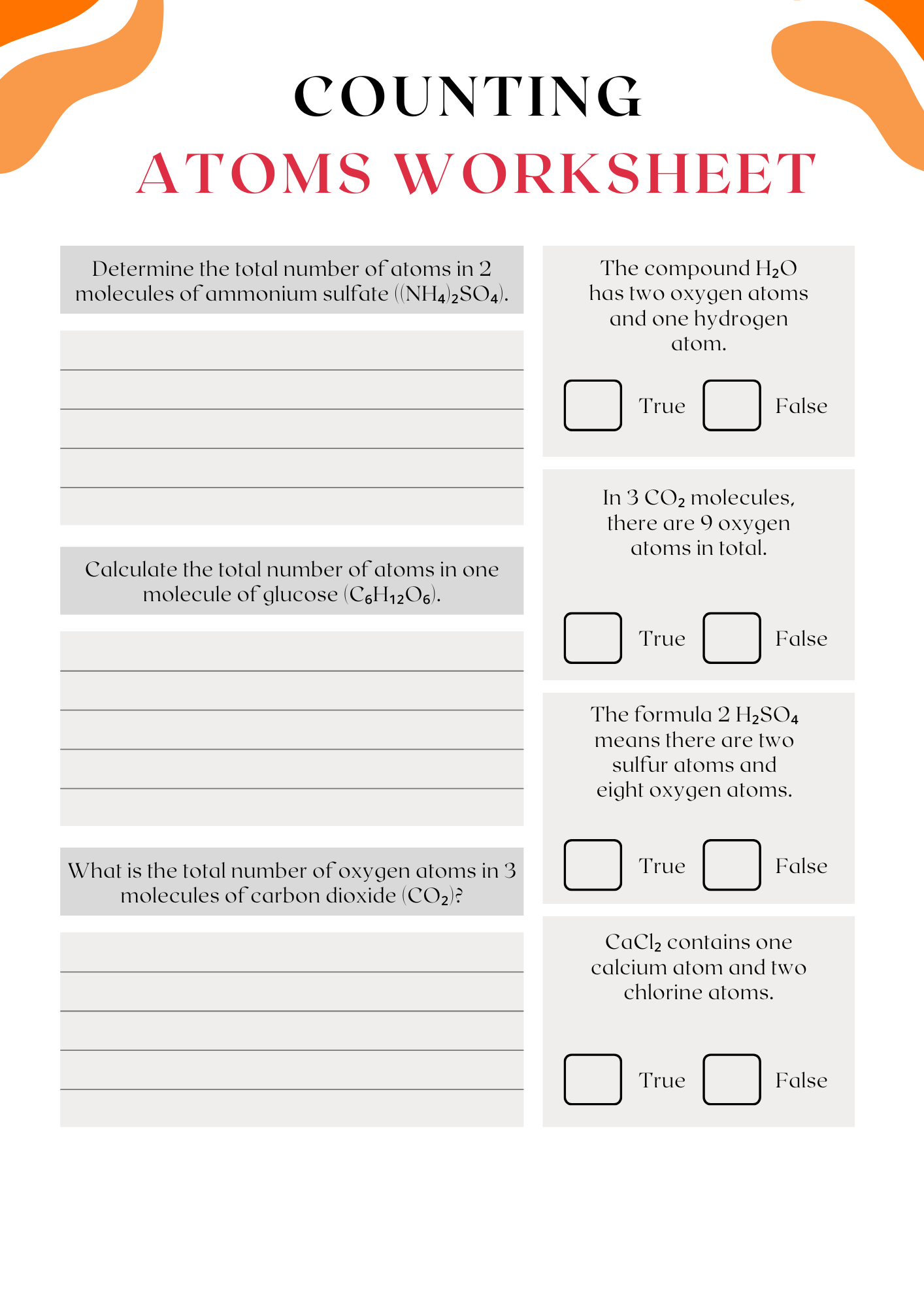
Elements Counting Atoms Worksheet
download now -
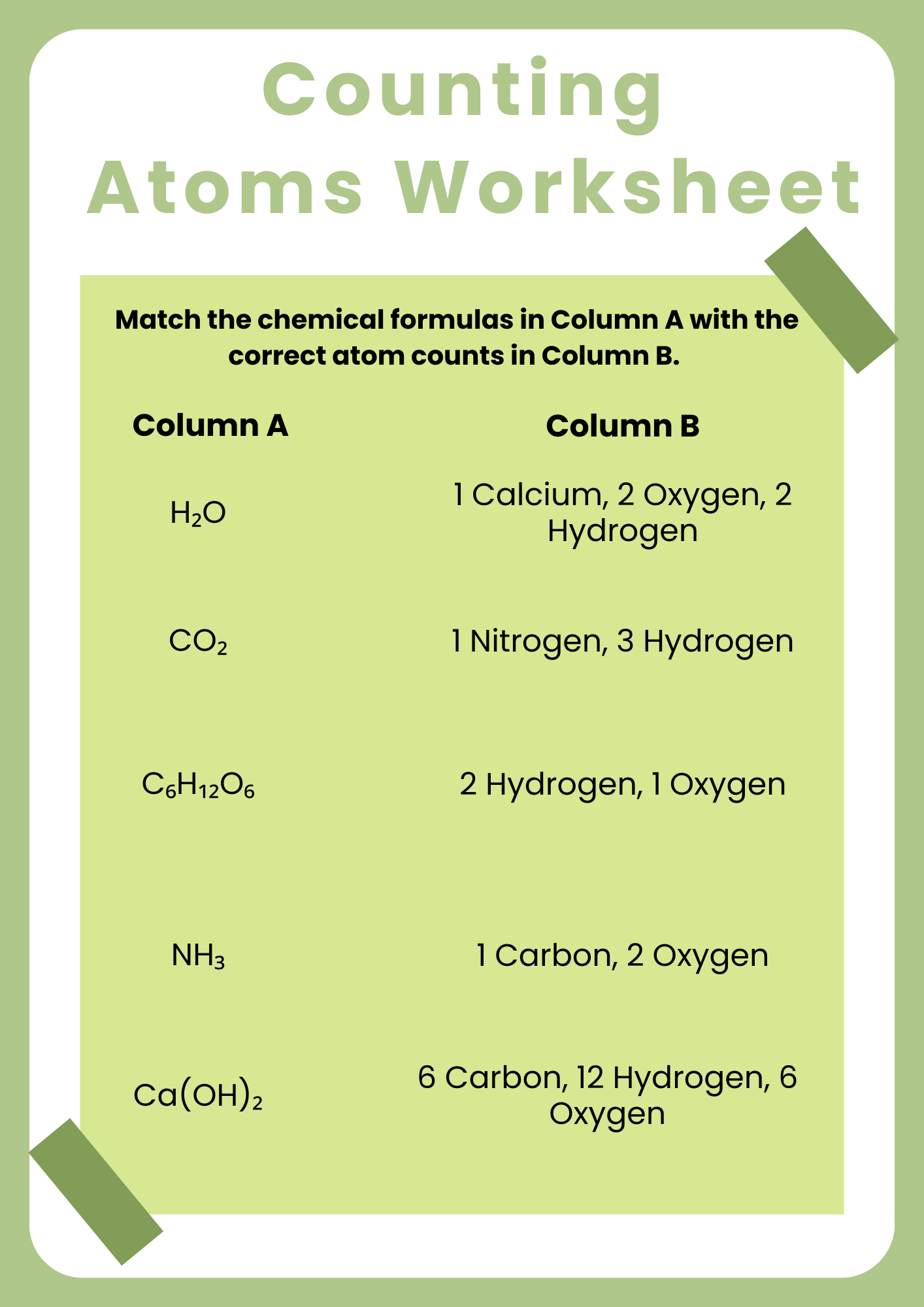
Compounds Counting Atoms Worksheet
download now -

Basic to Advanced Counting Atoms Worksheet
download now -
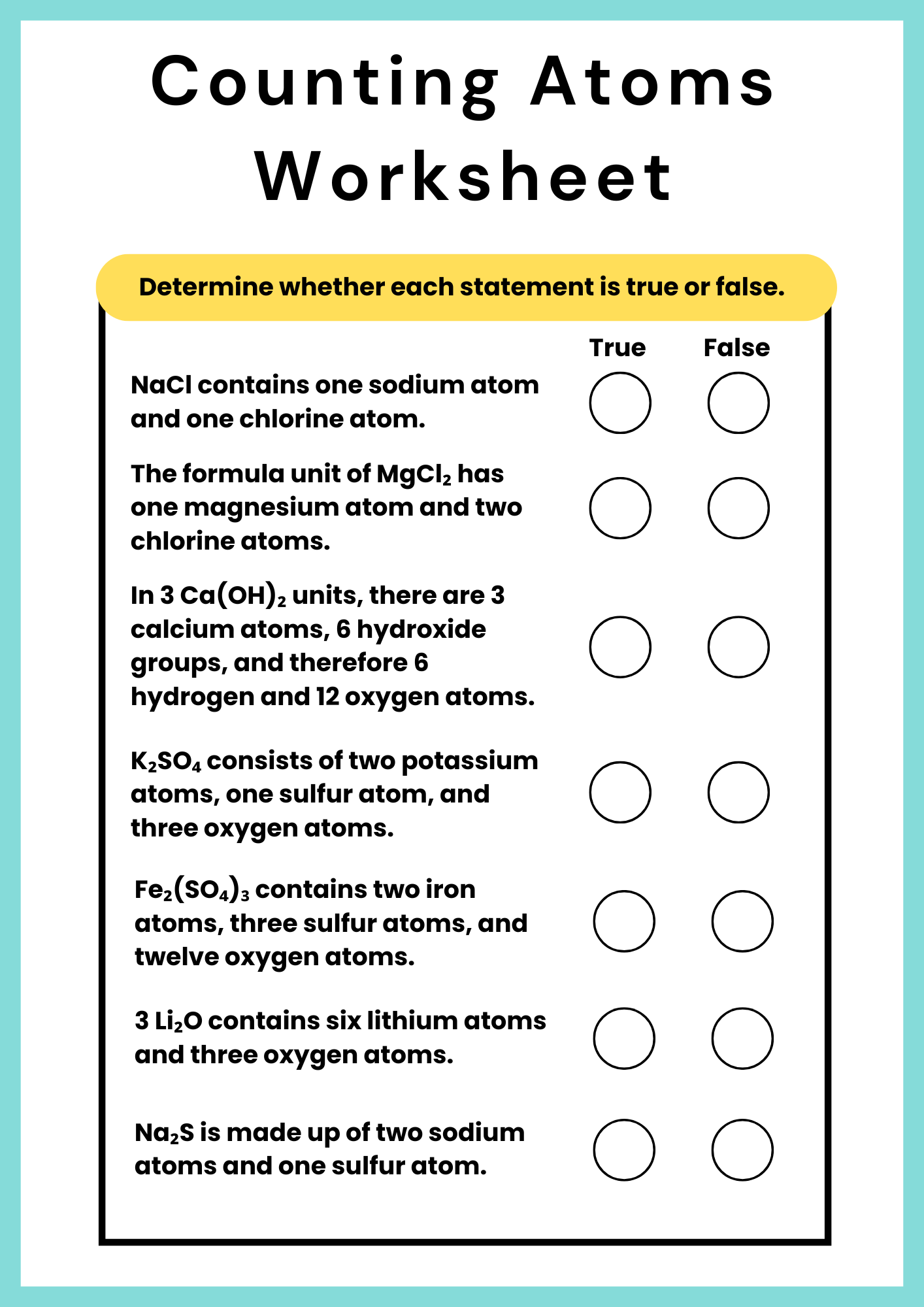
Ionic Counting Atoms Worksheet
download now -

Classroom Counting Atoms Worksheet
download now -
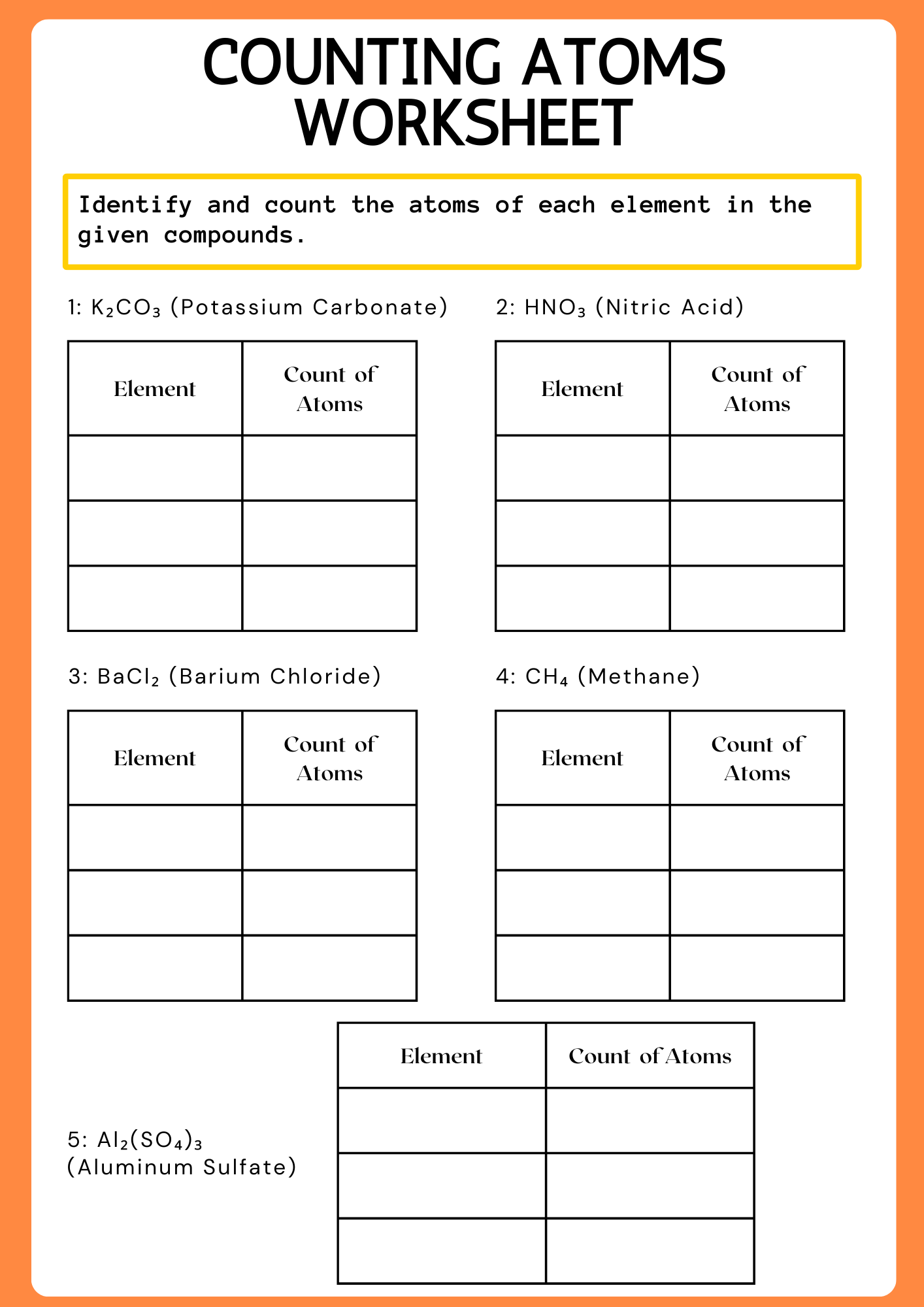
Counting Atoms Worksheet Mid School
download now -
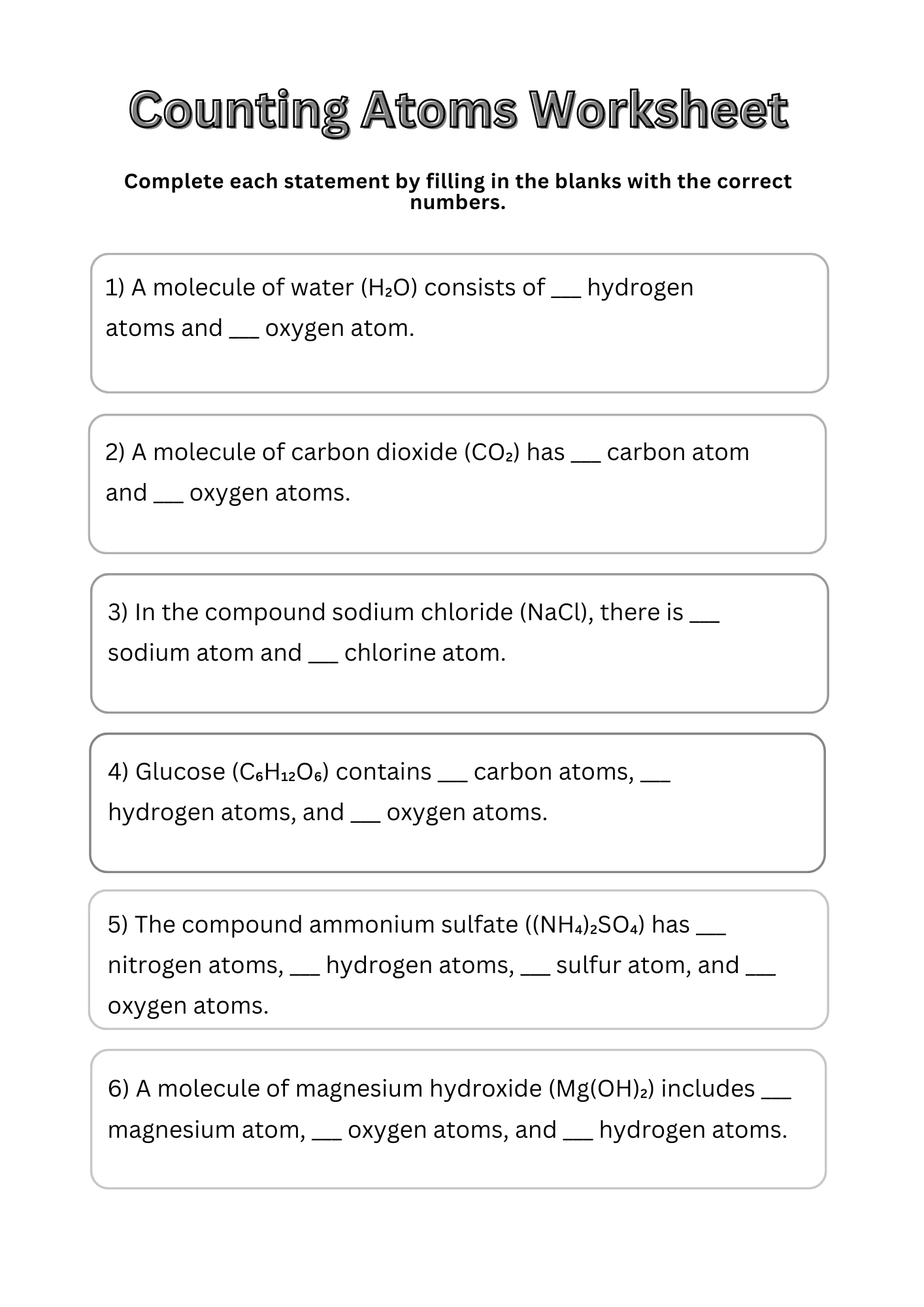
Counting Atoms Worksheet 8th Grade
download now -
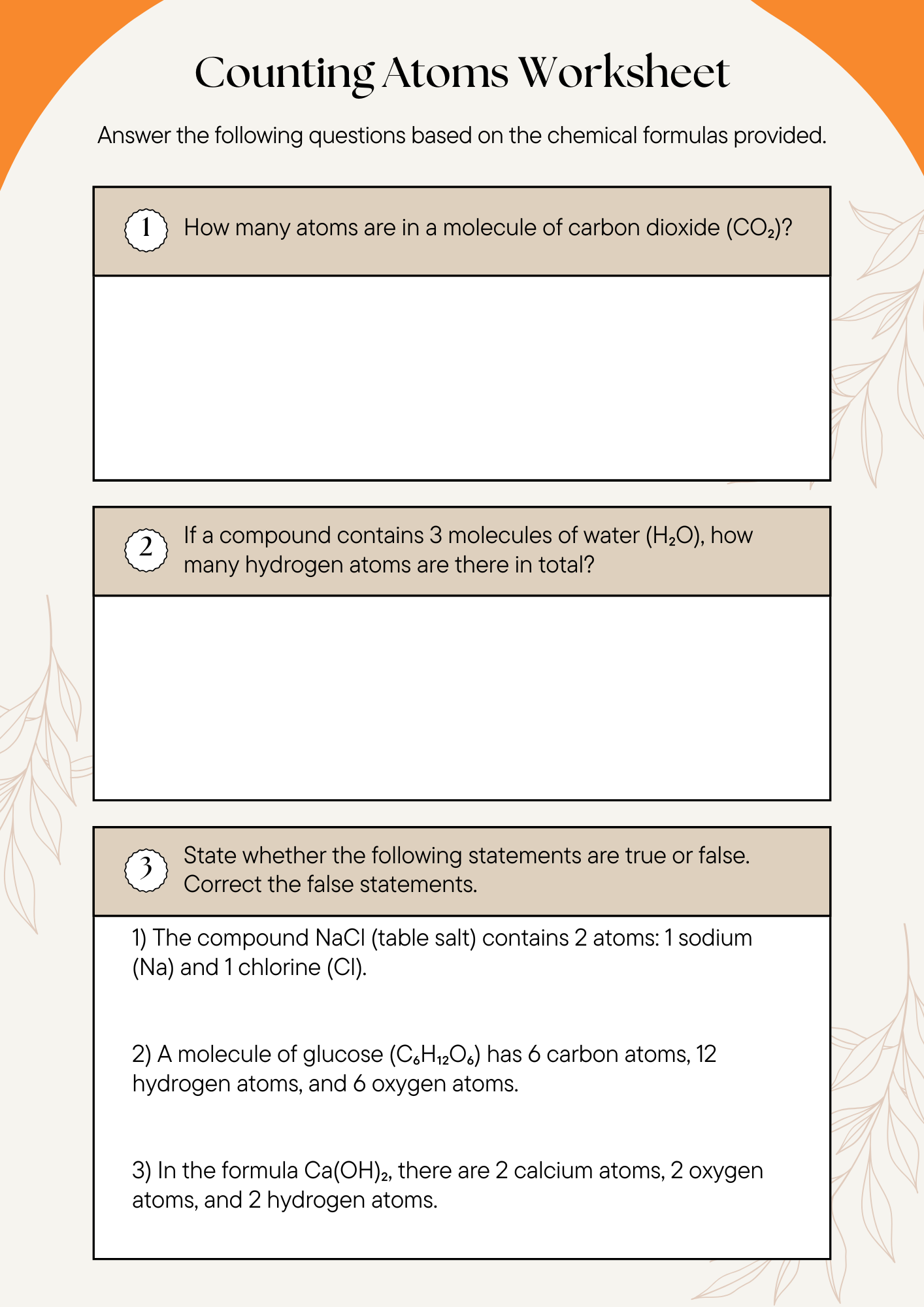
7th Grade Counting Atoms Worksheet
download now -

Counting Atoms Worksheet 9th Grade
download now -

Counting Atoms Worksheet for 10th Grade
download now -
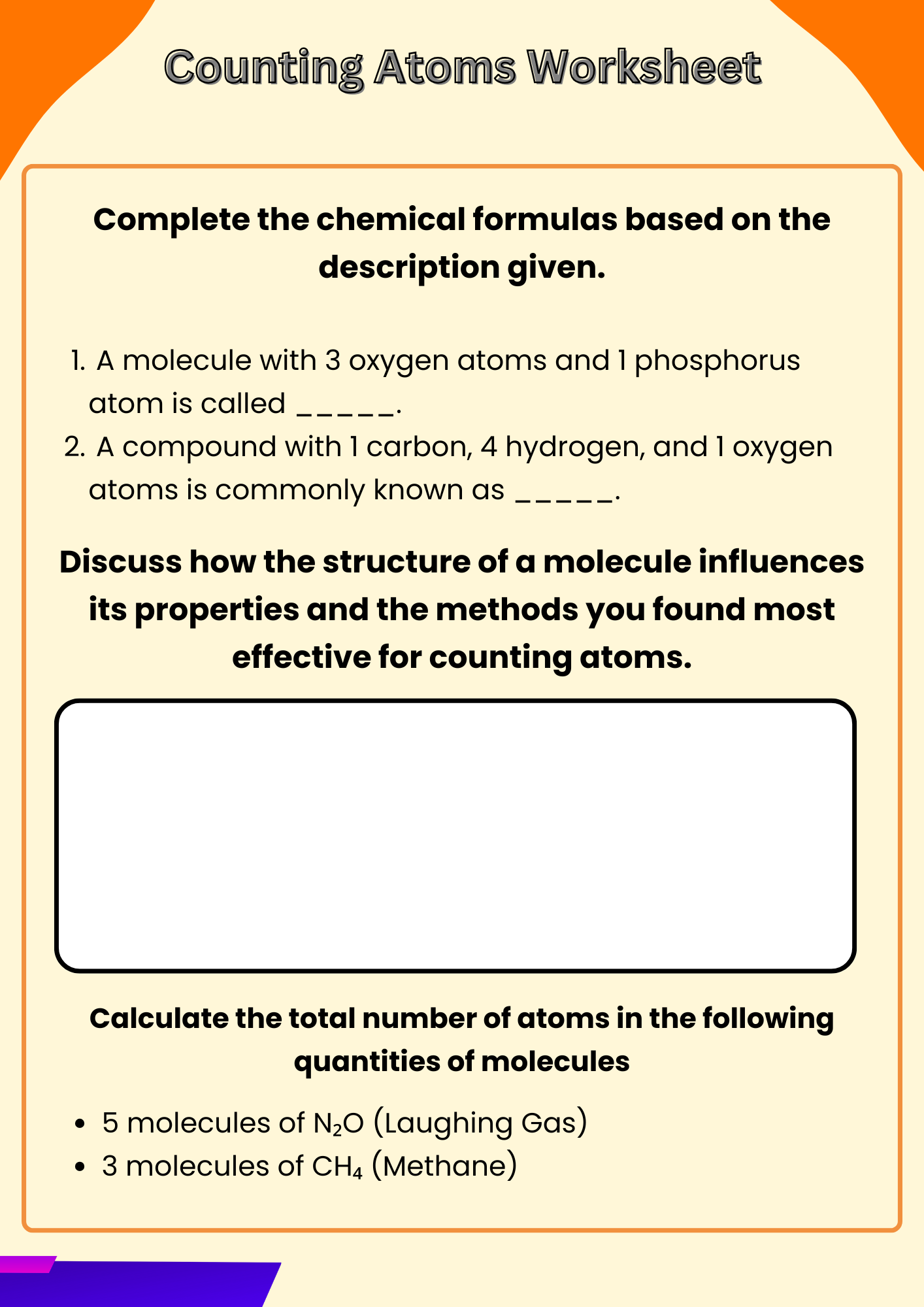
Molecule Counting Atoms Worksheet
download now -

Practice Counting Atoms Worksheet
download now -

Counting Atoms Worksheet for Educators
download now -
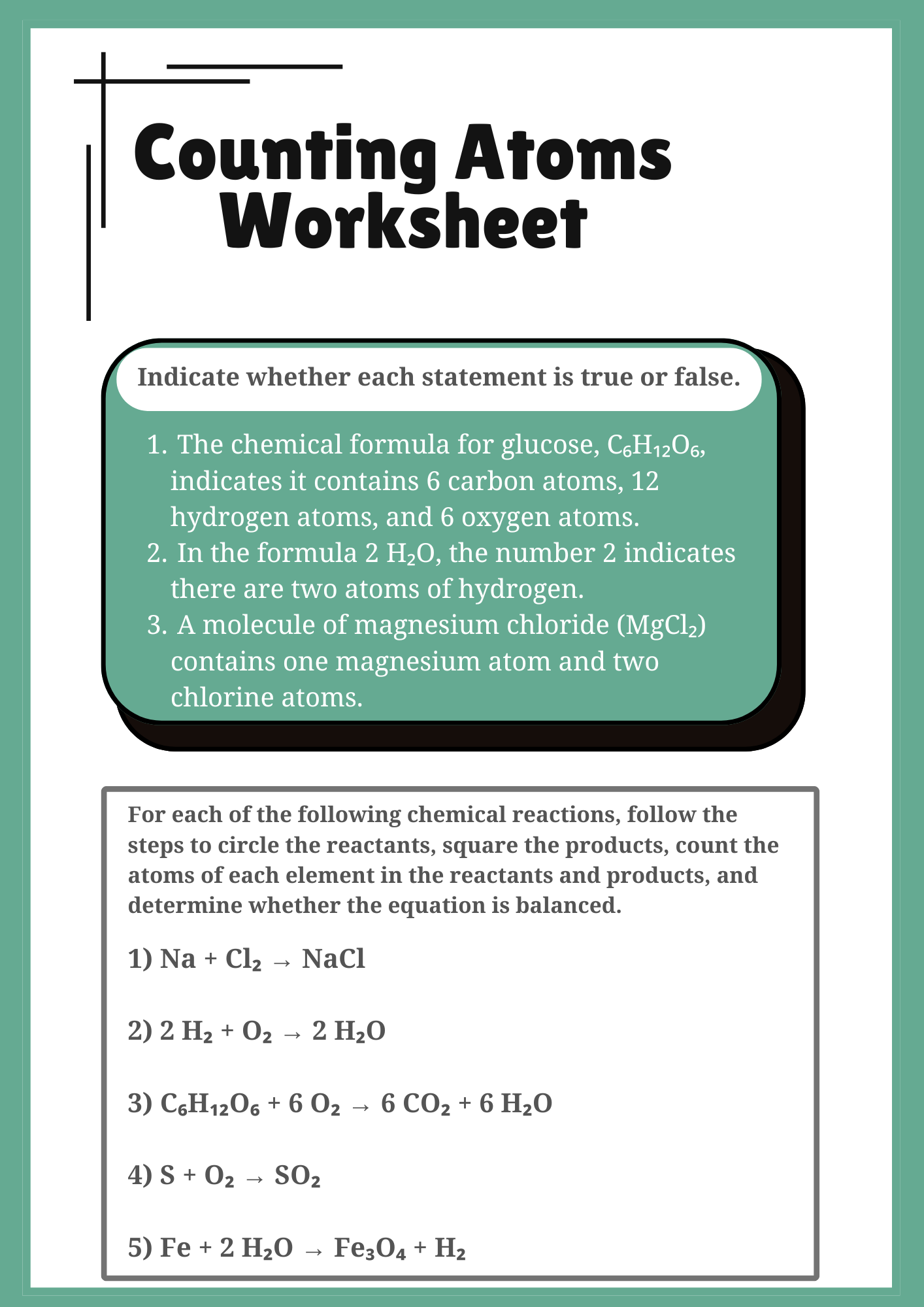
Interactive Counting Atoms Worksheet
download now -

Basic Counting Atoms Worksheet
download now -

Elementary School Counting Atoms Worksheet
download now -

Theoretical Counting Atoms Worksheet
download now -
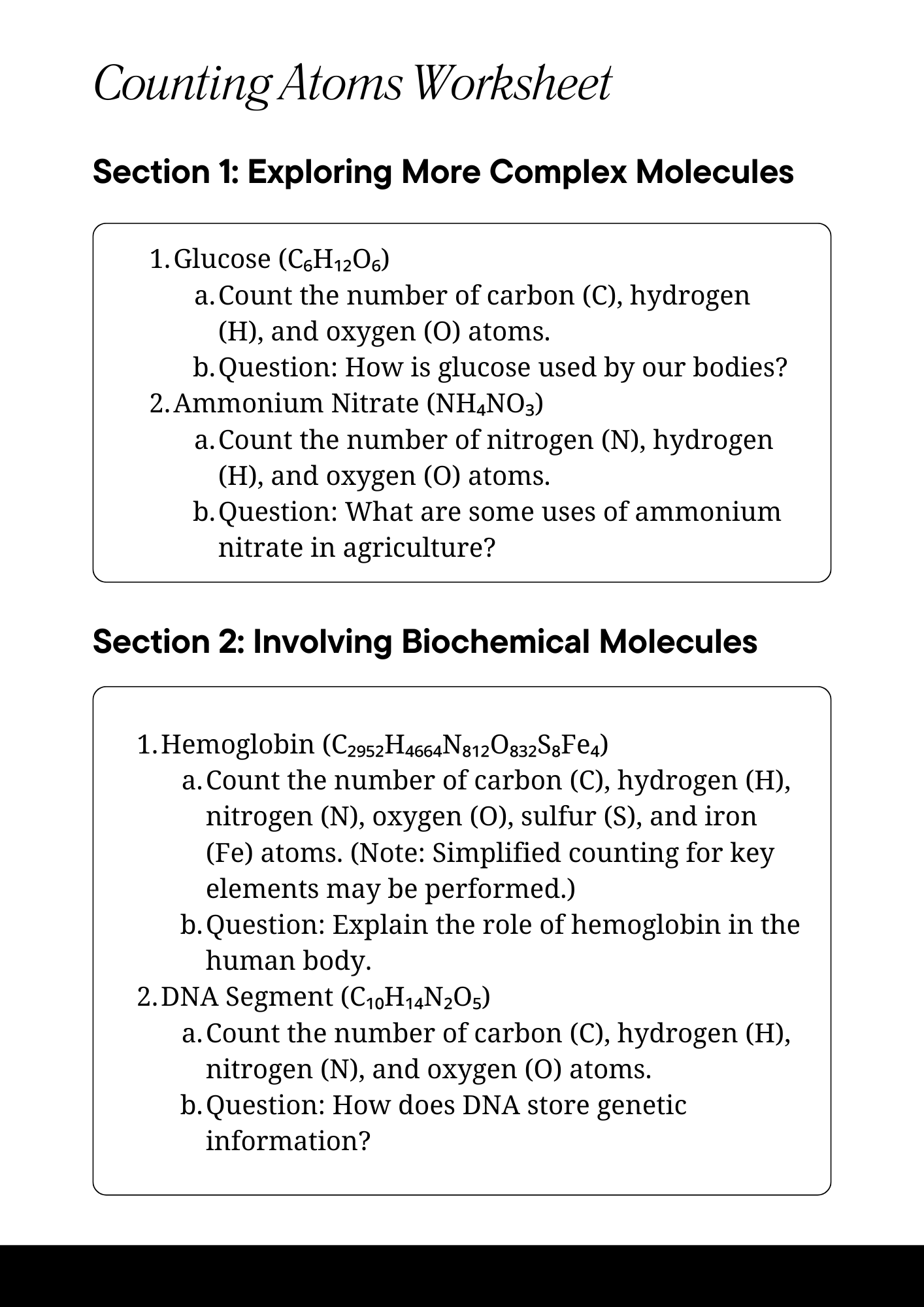
Science Counting Atoms Worksheet
download now -

Counting Atoms Worksheet for Beginners
download now -

Learning to Counting Atoms Worksheet
download now -
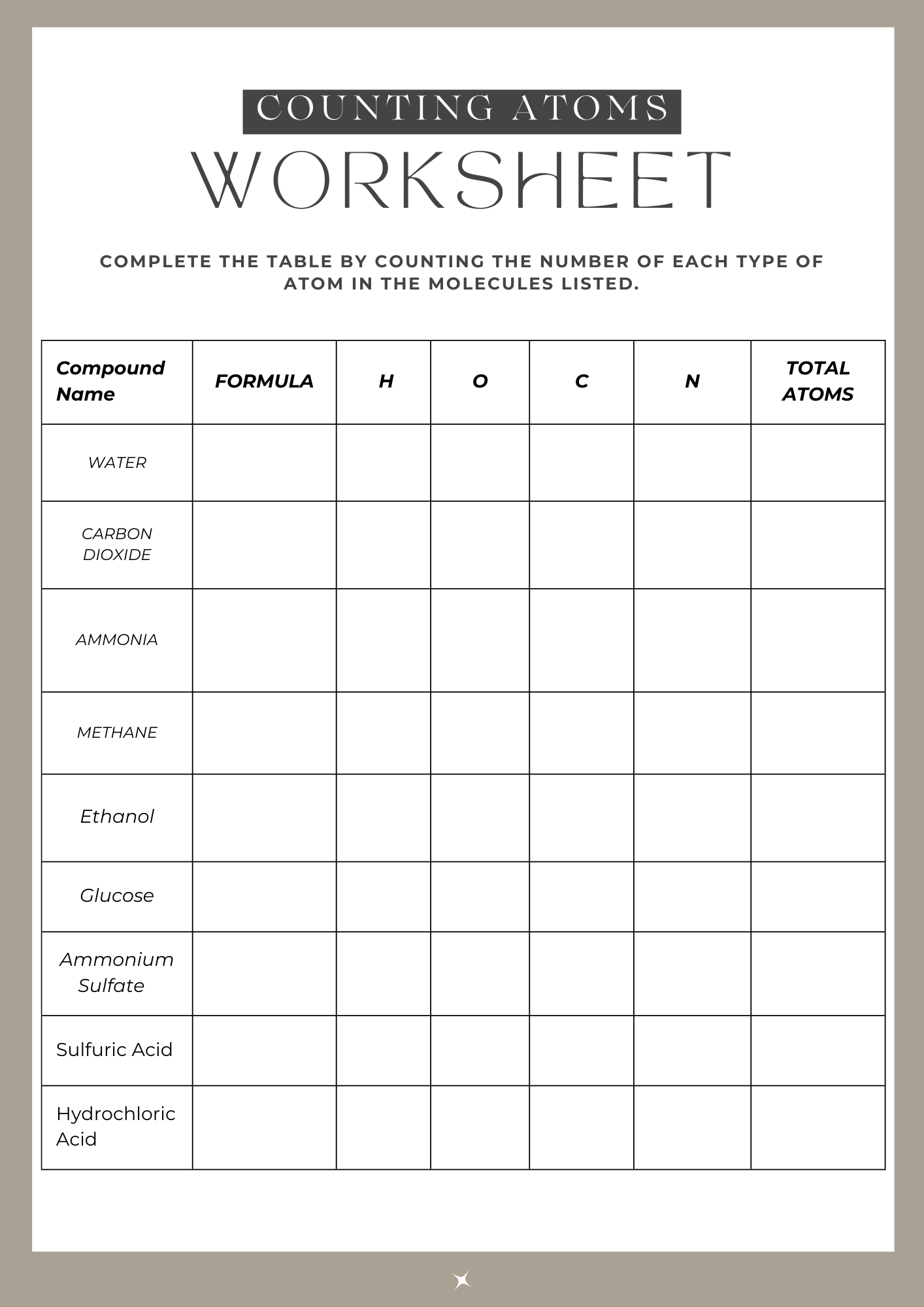
7.0.1 Counting Atoms Worksheet
download now -

Educational Counting Atoms Worksheet
download now -
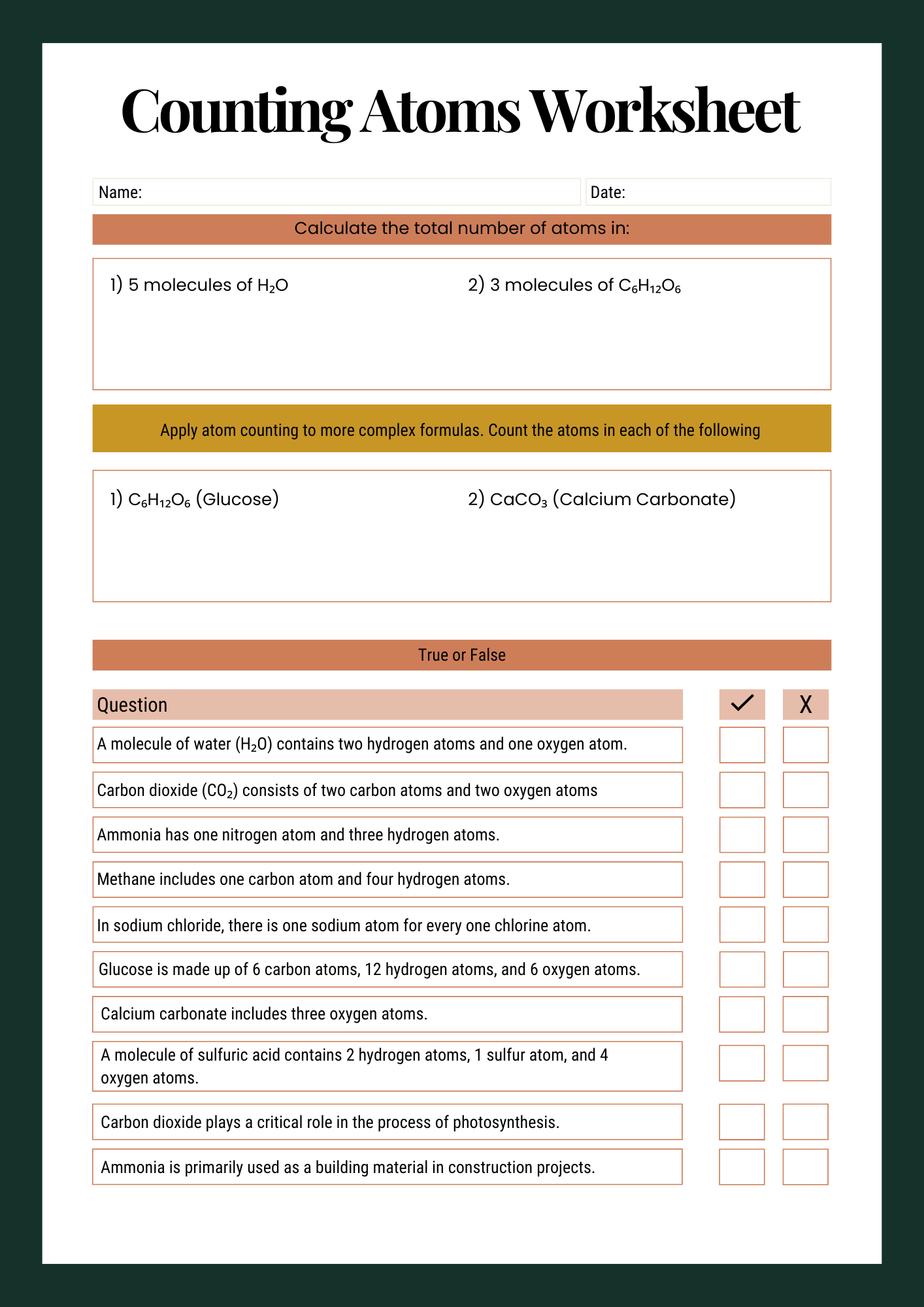
Counting Atoms Worksheet for Schools
download now -

Counting Atoms Worksheet for Teachers
download now -

Counting Atoms Worksheet for Students
download now -
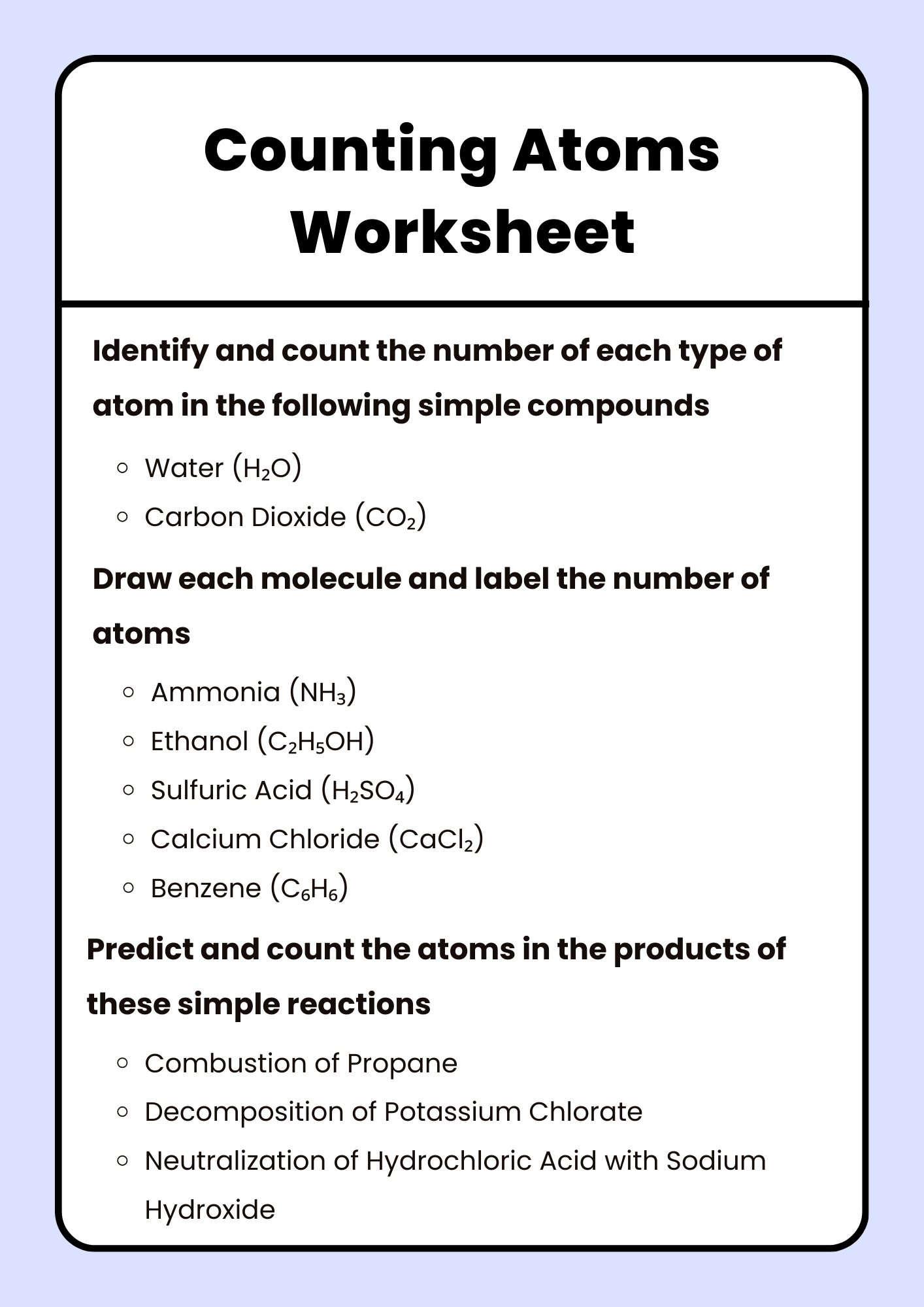
Counting Atoms Worksheet for Homeschool
download now -

Sample Counting Atoms Worksheet
download now -

Counting Atoms Worksheet for Homework
download now -
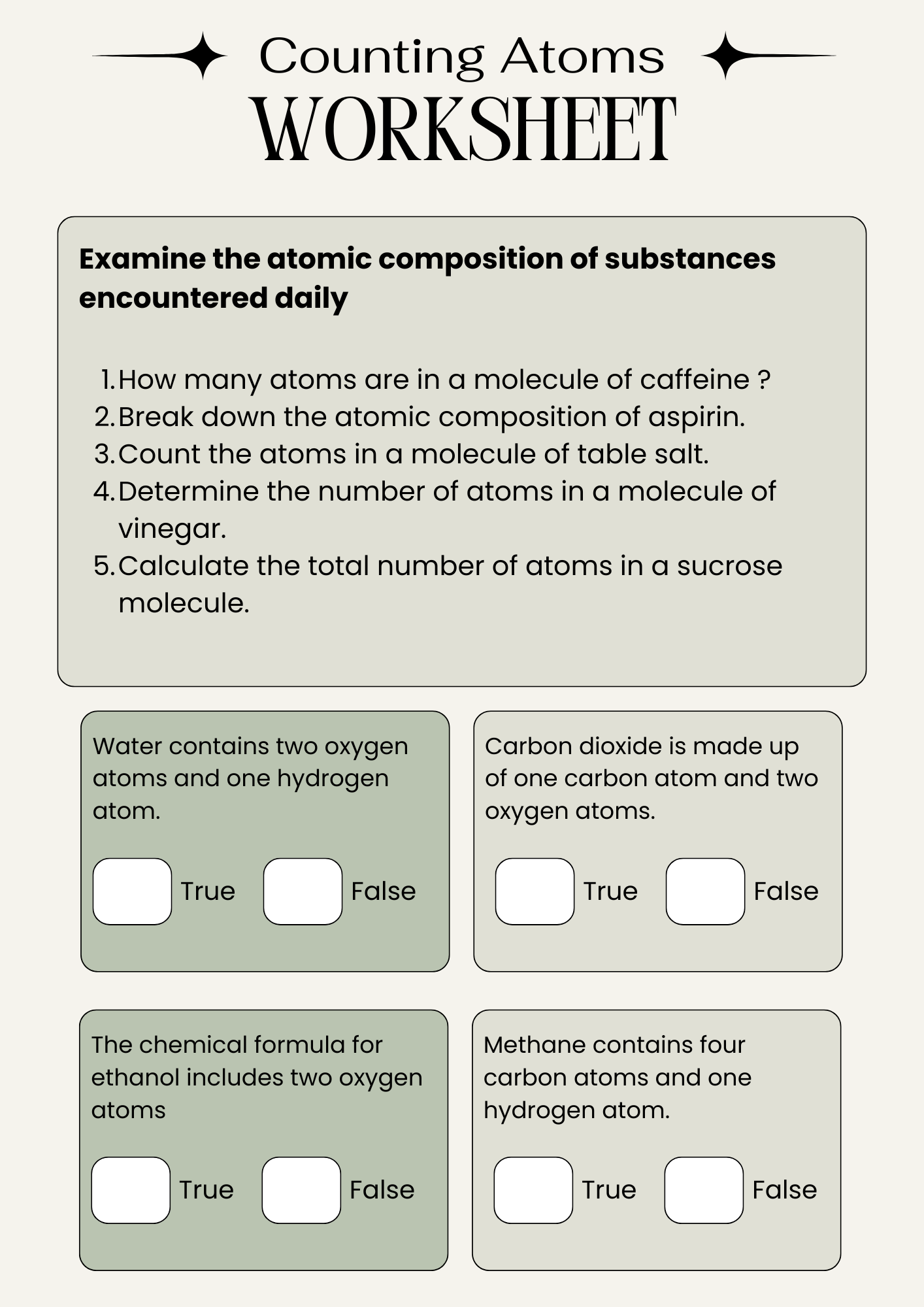
Comprehensive Counting Atoms Worksheet
download now -

Innovative Counting Atoms Worksheet
download now -

Counting Atoms Worksheet for Secondary Education
download now -
Counting Atoms Worksheet for Tutors
download now -

Counting Atoms Worksheet for Advanced Chemistry
download now -

Integration Counting Atoms Worksheet
download now -

Hard Counting Atoms Worksheet
download now -
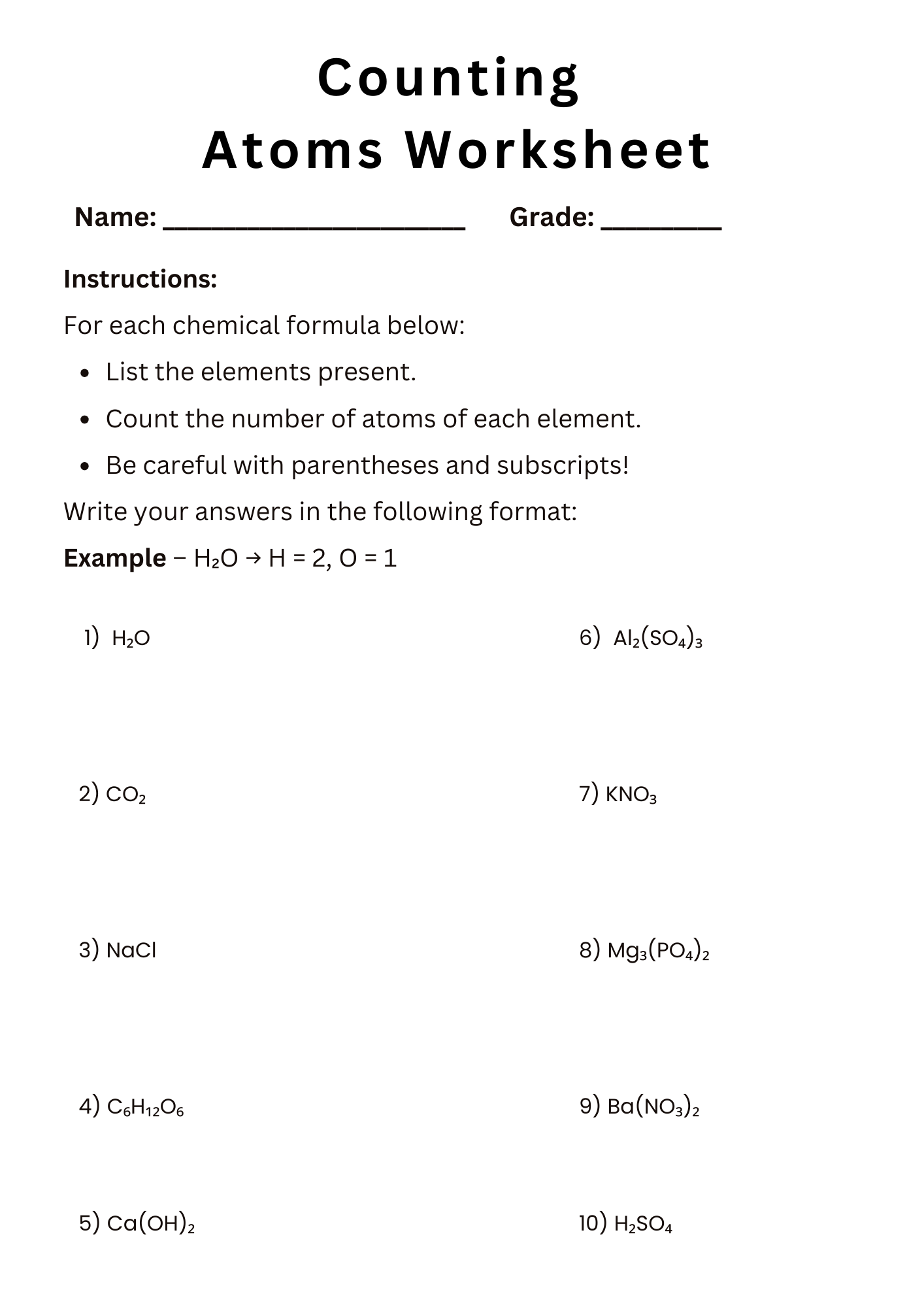
Counting Atoms Worksheet Answer Key
download now -

Middle School Counting Atoms Worksheet
download now -
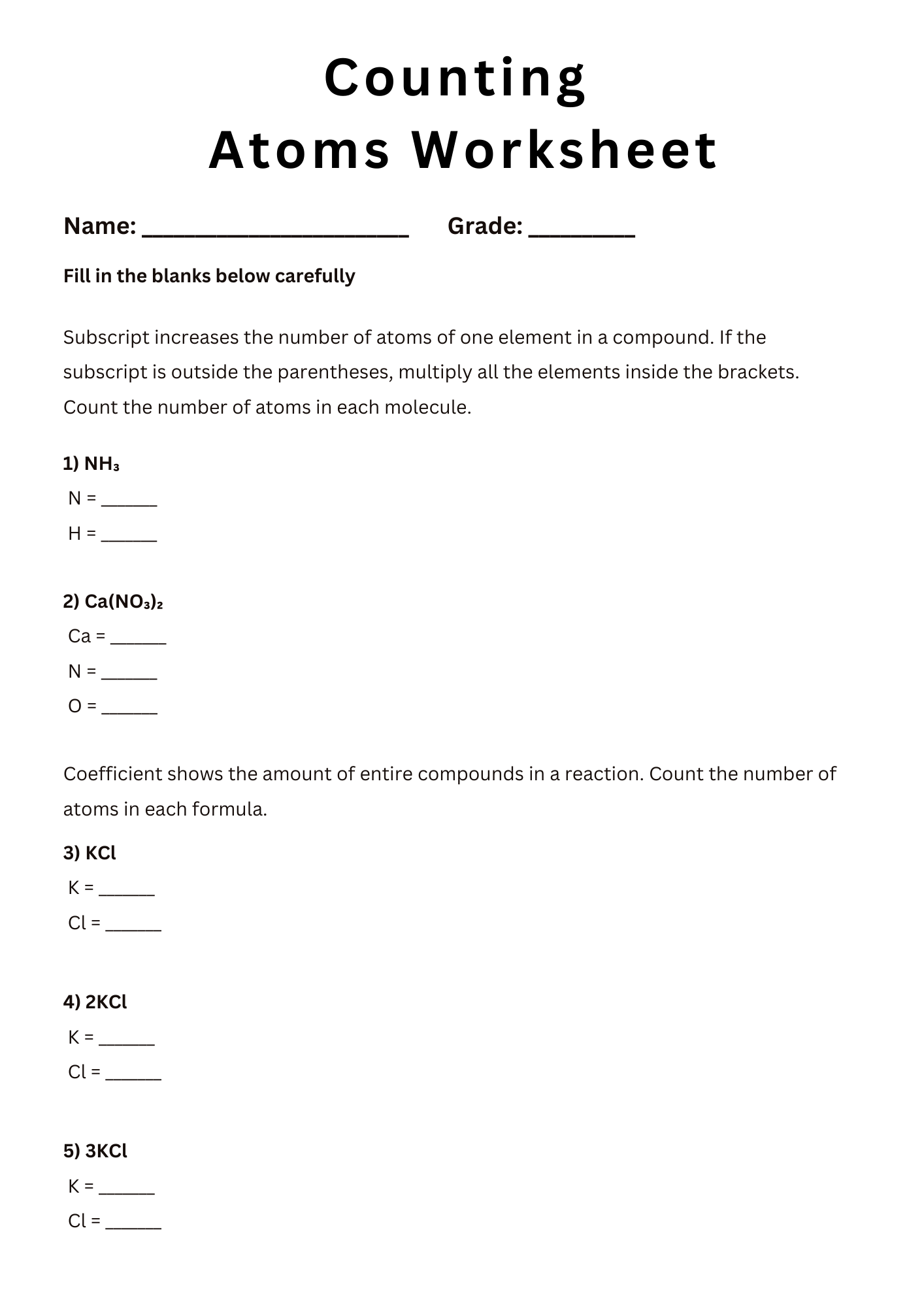
Counting Atoms in Formulas Worksheet
download now -
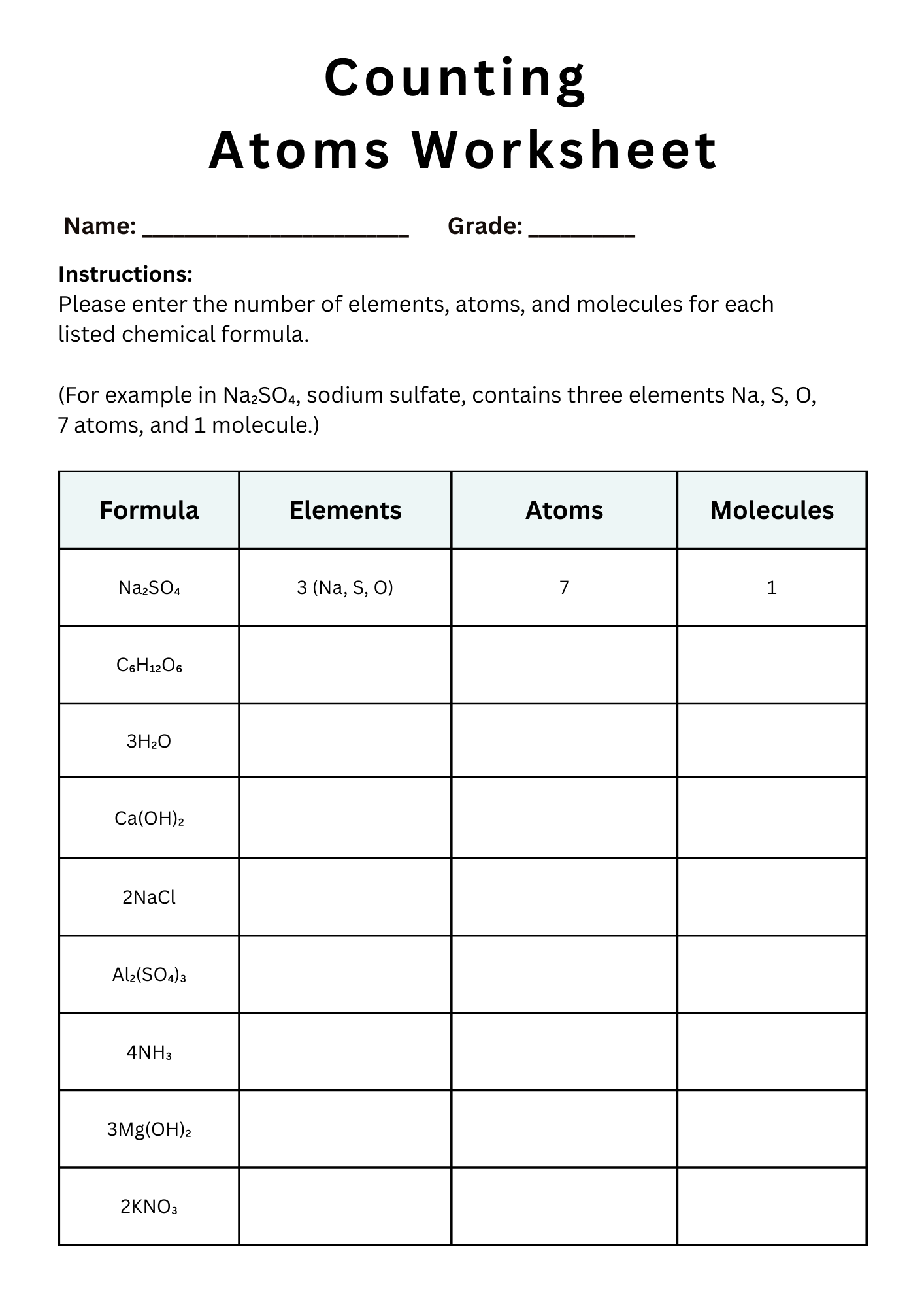
Counting Atoms Worksheet PDF
download now -

6th Grade Counting Atoms Worksheet
download now -
Blank Counting Atoms Worksheet
download now -
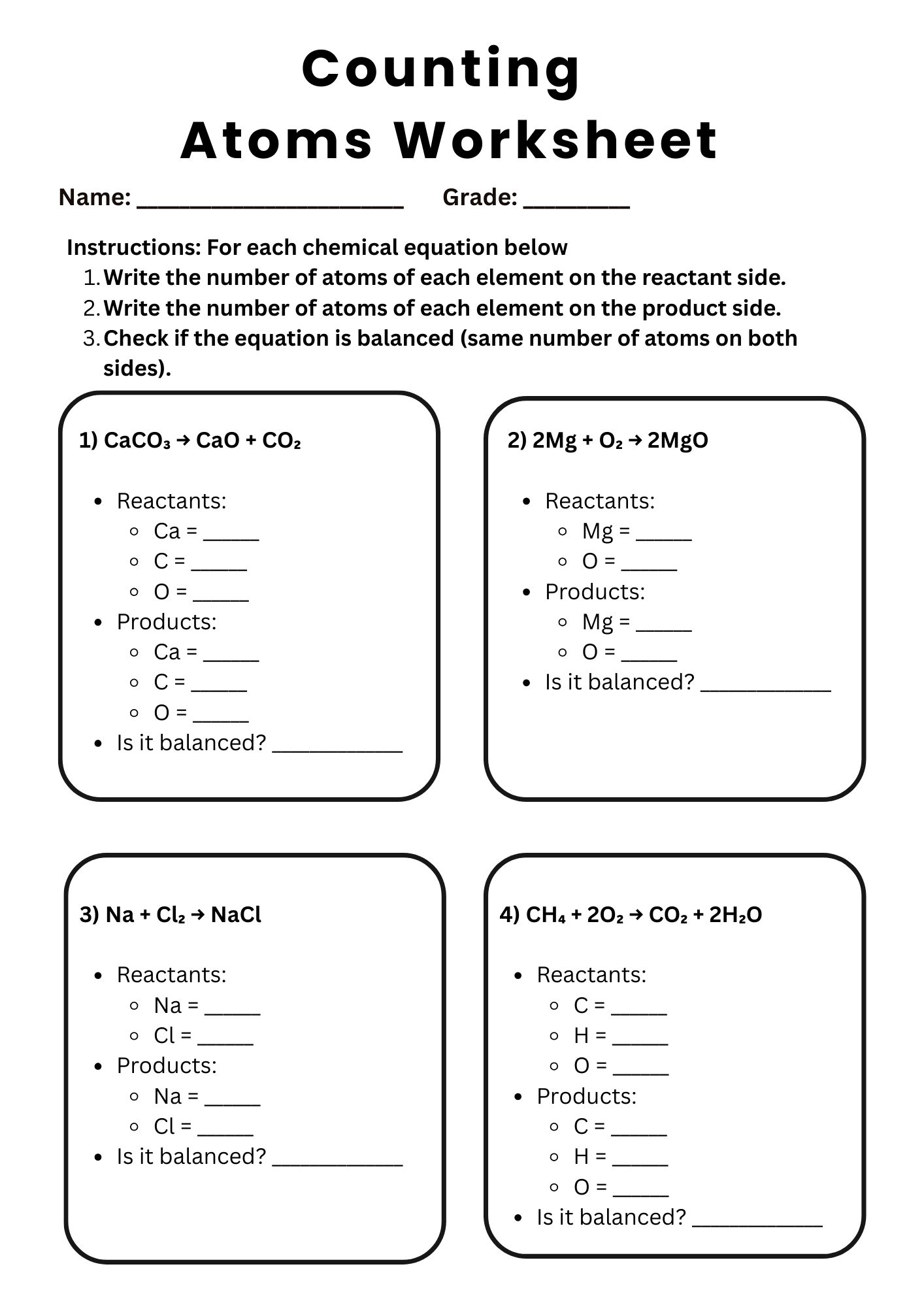
Counting Atoms Chemical Equations Worksheet
download now -

Counting Atoms Worksheet Answers
download now -

Magnesium Counting Atoms Worksheet
download now -
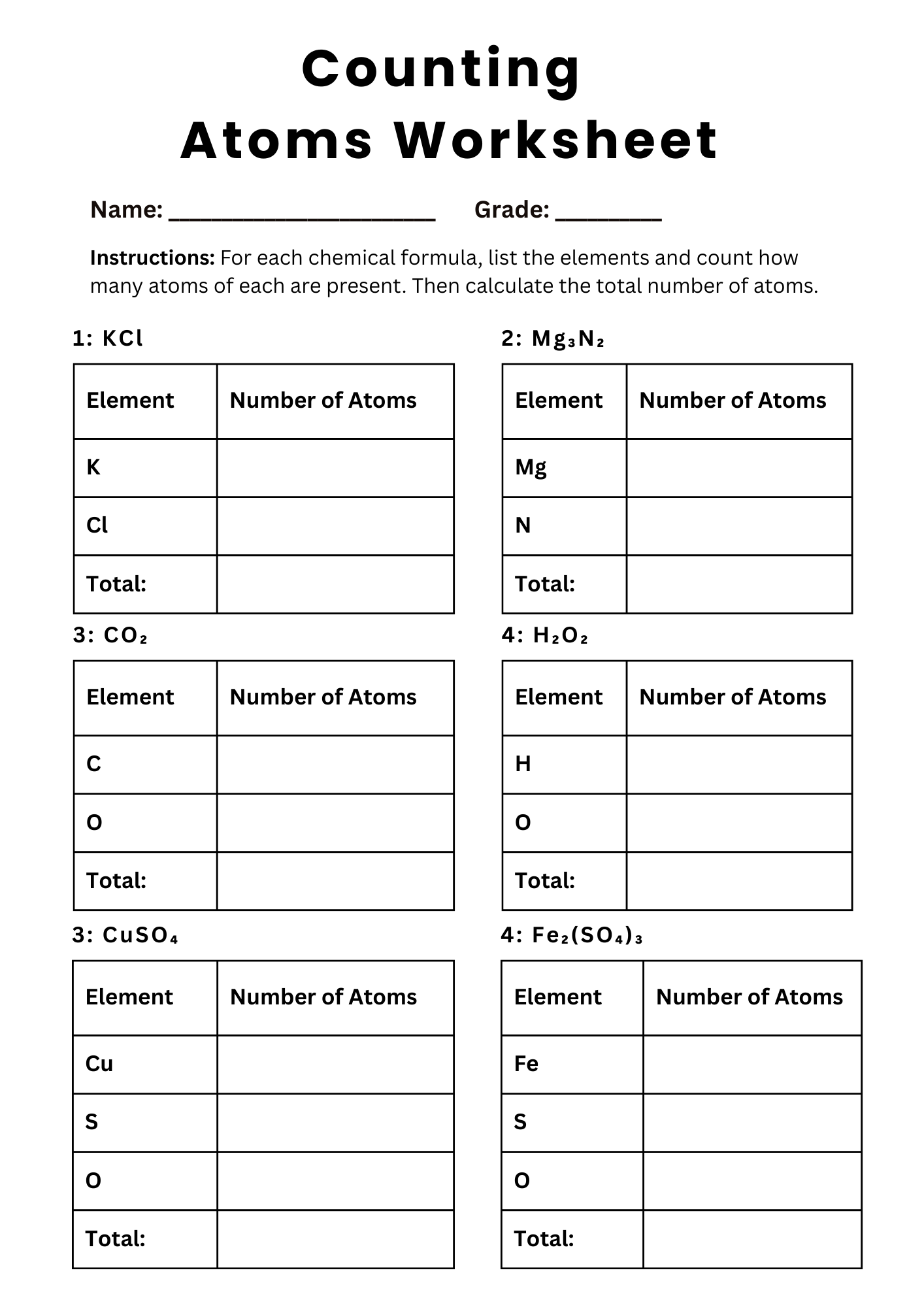
Counting Atoms Number Worksheet
download now -

Counting Atoms Practice Problems Worksheet
download now -

Counting Atoms Classwork Worksheet
download now -

Counting Atoms Maze Worksheet
download now
What is a Counting Atoms Worksheet?
A Counting Atoms Worksheet is an educational tool used primarily in chemistry classes to help students learn how to determine the number of each type of atom in chemical compounds. The worksheet typically includes a variety of exercises where students analyze chemical formulas to count and record the number of individual atoms or types of atoms present. These worksheets are designed to improve students’ understanding of molecular composition and enhance their ability to interpret and balance chemical equations. They are essential for developing a solid foundation in chemical stoichiometry and for helping students visualize how atoms combine to form different substances.
Why Use Counting Atoms Worksheet?
✔️ Reinforces Chemical Concepts: It helps students understand the basic building blocks of chemical compounds by visualizing and quantifying the elements involved.
✔️ Develops Analytical Skills: Counting atoms in various chemical formulas enhances critical thinking and analytical skills, crucial for problem-solving in chemistry.
✔️ Aids in Balancing Equations: Mastery of atom counting is essential for balancing chemical equations, a fundamental skill in chemistry that ensures the conservation of mass in reactions.
✔️ Enhances Memorization of Formulas: Regular practice with these worksheets helps students memorize common chemical formulas and their corresponding molecular structures.
✔️ Prepares for Advanced Topics: It lays a solid foundation for more complex topics in chemistry, such as stoichiometry and thermodynamics, by ensuring students understand the composition of molecules.
✔️ Interactive Learning Tool: These worksheets provide an interactive and engaging way for students to learn, making abstract concepts more tangible and easier to understand.
Step-by-Step Guide to Counting Atoms in Chemical Compounds
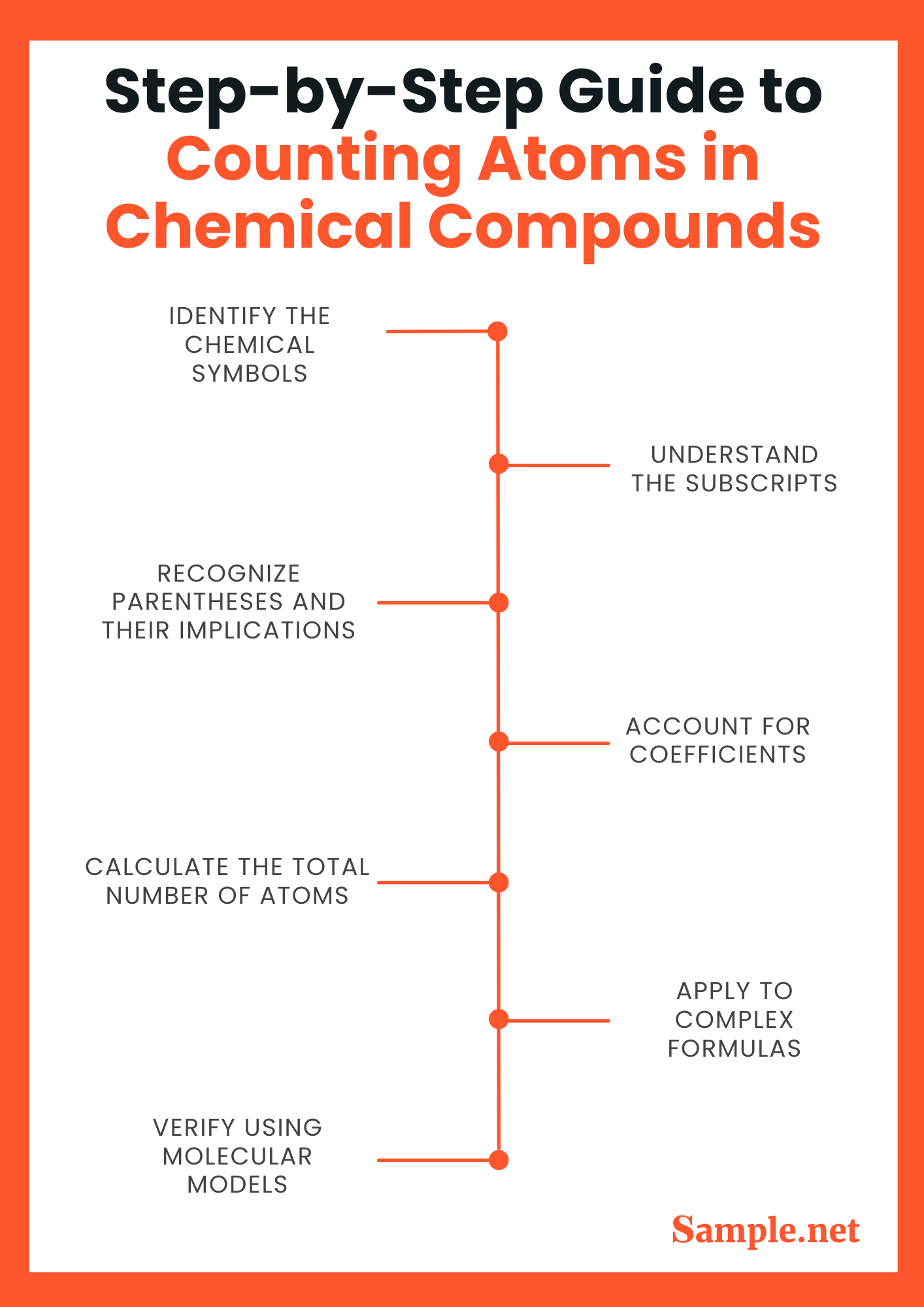
Step 1: Identify the Chemical Symbols
- Explanation: Each element in a compound is represented by a chemical symbol, such as H for Hydrogen or O for Oxygen. First, identify these symbols in the chemical formula to know which elements are present.
Step 2: Understand the Subscripts
- Explanation: Subscripts following the chemical symbols indicate the number of atoms of each element in one molecule of the compound. For example, in H₂O, the subscript ‘2’ next to H means there are two hydrogen atoms.
Step 3: Recognize Parentheses and Their Implications
- Explanation: Parentheses in chemical formulas are used when a group of atoms is a part of the compound more than once. For instance, in Ca(OH)₂, the (OH)₂ indicates that the hydroxide group (OH) repeats twice, containing two oxygen and two hydrogen atoms in total.
Step 4: Account for Coefficients
- Explanation: Coefficients before a chemical formula represent the number of identical molecules. If a formula like 3H₂O is given, it means there are three molecules of water, leading to a total of six hydrogen atoms and three oxygen atoms.
Step 5: Calculate the Total Number of Atoms
- Explanation: Multiply the subscripts by the coefficients (if present) for each element to get the total number of atoms of each type in the molecule. Sum all atoms together for the total atomic count in the molecule.
Step 6: Apply to Complex Formulas
- Explanation: For more complex formulas, especially those involving multiple polyatomic ions or different groups, repeat the steps for each segment of the molecule and add up the totals for each element separately.
Step 7: Verify Using Molecular Models (Optional)
- Explanation: To aid in understanding and to verify your atom counting, consider constructing a molecular model using available tools or kits. This visual aid can help confirm that your atom counts are correct and provide a tangible way to see the molecular structure.
How to Use a Counting Atoms Worksheet?
1️⃣ Distribute the Worksheet: Begin by providing each student with a copy of the Counting Atoms Worksheet. Ensure that the worksheet includes a variety of chemical formulas ranging from simple to complex.
2️⃣ Review Chemical Symbols and Subscripts: Before starting, review the basic concepts of chemical symbols and subscripts with the students. Explain that each symbol represents an element and the subscript shows the number of atoms of that element in the molecule.
3️⃣ Discuss the Significance of Parentheses and Coefficients: Clarify how parentheses are used to indicate groups of atoms that are together in a molecule, and how coefficients outside the parentheses affect the count of all atoms inside the parentheses.
4️⃣ Work Through Examples Together: Solve one or two examples as a class to demonstrate how to count atoms. For instance, break down the formula for a complex molecule step by step, showing how to apply subscripts and coefficients.
5️⃣ Independent or Group Practice: Allow students to work independently or in small groups to complete the worksheet. Encourage them to discuss their strategies and findings with their peers to enhance understanding.
6️⃣ Review and Discuss Answers: After completing the worksheet, review the answers as a class. Discuss common mistakes and correct them, reinforcing the correct methods for counting atoms. This step is crucial for solidifying understanding and correcting misconceptions.
